

Tours diarios en Grupo y Privado
Salidas desde Mérida Yucatán

Descubre las Maravillas de Yucatán

La Mejor Experiencia al Viajar

Tenemos los Mejores Tours en Grupo Todos los días
Bienvenidos a viajes colibrí yucatán.
Organizamos tours en grupo todos los días a diferentes destinos de Yucatán. Tenemos tours todos los días desde Mérida. Realizamos tours personalizados de acuerdo a las necesidades e intereses de cada persona o grupo, contáctanos para apoyarte a buscar el mejor tour para ti y tu familia o amigos.

4.9 Calificación
En base a más de 1,000 comentarios registrados en Google Maps, Facebook, Trip Advisor, Get Your Guide y más de 10,000 viajeros satisfechos que han realizado tours con nosotros.

La mejor transportación
Somos una agencia de viajes local que cuenta con vehículos modernos, cómodos y limpios para todas las capacidades: desde autos, camionetas y autobuses.

Pago Seguro
Reserva a través de nuestra página web de forma segura, se aceptan todas las tarjetas de débito y crédito. Si deseas reservar por transferencia, contáctanos al (+52) 999 204 5234, con gusto uno de nuestros asesores te proporcionará los datos y te apoyará con tu reservación.

Los Mejores Guías
Viaja con los mejores guías del estado para que disfrutes de la mejor experiencia, en compañía de guías certificados que te brindarán la información oportuna sobre los lugares a visitar y te apoyarán con las fotos durante el tour.


Pago en Tarjeta (Parcial o Total)
Al momento de realizar tu pago en nuestra página web, puedes elegir reservar cualquier tour con un anticipo de la mitad del costo del tour y pagar el saldo en efectivo al abordar el día de cada tour reservado. Igualmente puedes elegir la opción de realizar el pago completo del tour.

Facturamos todos los servicios
Si necesitas factura envíamos un correo con tu constancia fiscal y cupón de reservación, es importante que la factura se solicite dentro del mes en el cual se realizó el pago del servicio. [email protected].
LOS 3 TOURS MÁS POPULARES
Te presentamos los 3 Tours más solicitados por los viajeros con los lugares más importantes que no te puedes perder en tu próxima visita a Mérida Yucatán
TOUR CHICHÉN ITZÁ PREMIUM
Tour coloradas plus, tour valladolid premium, las mejores vacaciones en mérida yucatán.
Deja en nuestras manos el disfrute de tu próximo viaje para conocer los lugares más bellos y representativos de Yucatán, no te preocupes por nada más que por divertirte y crear los mejores recuerdos en compañía de los mejores operadores y guías certificados.
Personaliza tu Viaje
Información relevante.
Aquí podrás encontrar información que puede ayudarte a organizar tu próximo viaje a Mérida Yucatán. Lugares interesantes que visitar, eventos culturales, promociones, hoteles, restaurantes y mucho más.

Los Mejores Tours en Mérida Yucatán 2024 (3 días de tours)
TOURS DESDE MÉRIDA YUCATÁN Planear las vacaciones en Mérida es una tarea importante si deseas conocer lo mejor, ya que hay muchos lugares hermosos que visitar; sin embargo en base …

Temporada de Flamencos Rosados en Yucatán (Con Fotos)
¿Cuándo y dónde se ven los Flamencos en Yucatán? (Con Fotos) Mucho se habla de la temporada de flamencos en Yucatán, principalmente la temporada de flamencos en Celestún ya que …
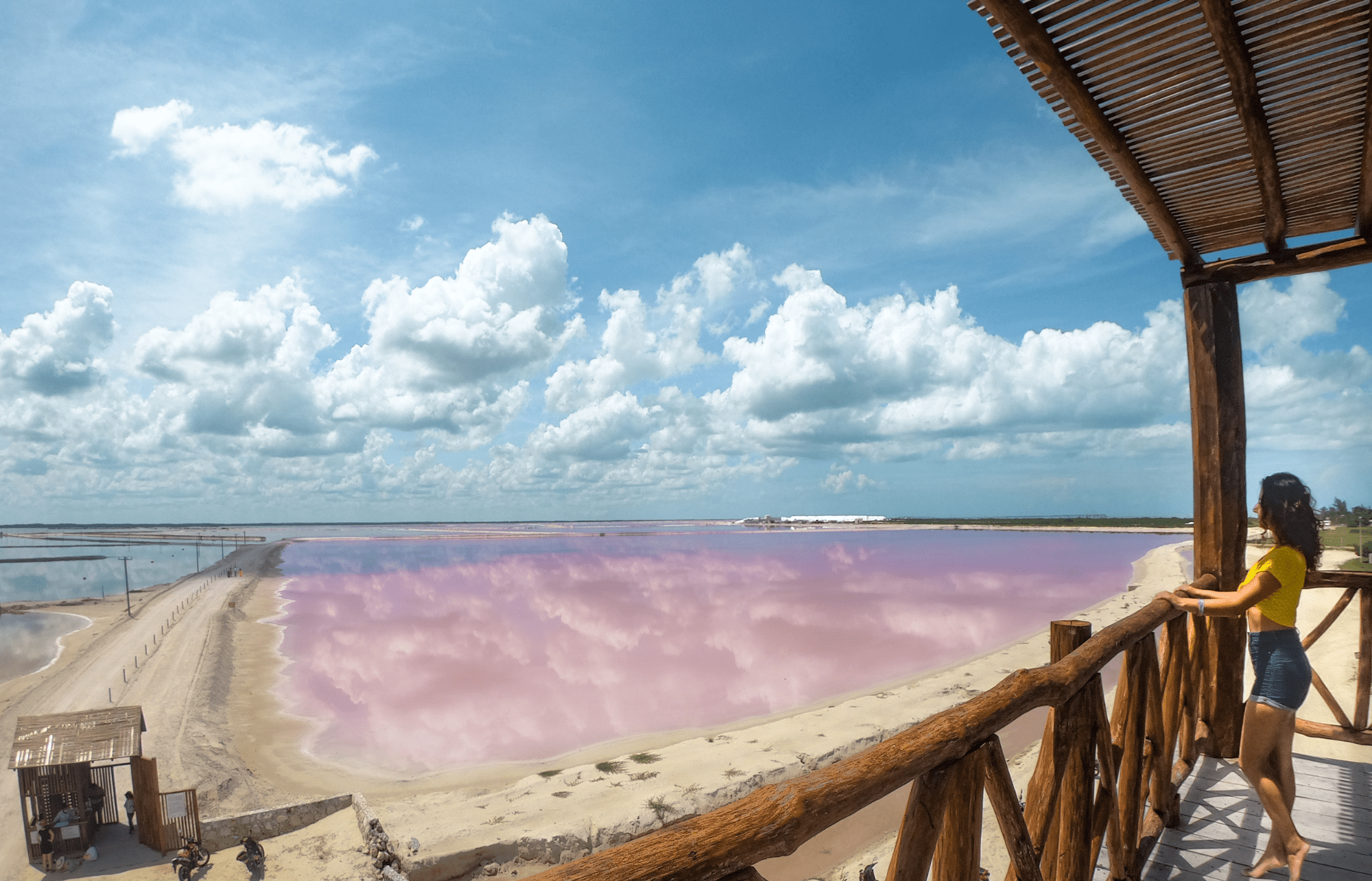
¿Cuándo están rosas Las Coloradas de Yucatán?
¿Cuándo están rosas Las Coloradas de Yucatán? ¿Por qué el agua es rosa? Este peculiar color se debe a las altas concentraciones de microorganismos halófilos, que son pequeñas bacterias que …
Busca los mejores Hoteles – Booking.com

IMAGES
COMMENTS
WEBLas Mejores vacaciones en Mérida Yucatán. Deja en nuestras manos el disfrute de tu próximo viaje para conocer los lugares más bellos y representativos de Yucatán, no te …