Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Find in topic

RELATED TOPICS
INTRODUCTION
The evaluation of fever in returned travelers should focus on the possible infections given the patient's clinical findings, travel geography, administration (if any) of vaccinations and malaria chemoprophylaxis, the nature and timeframe of potential exposure(s), and the incubation period(s) of the relevant possible infections ( table 1 ) [ 1,2 ].
Good resources that provide current information about the infections that occur in various geographic areas are essential [ 3-5 ]. The United States Centers for Disease Control and Prevention website includes an online version of Health Information for International Travel under Travelers' Health and updates on travel-related infections [ 5 ]. The World Health Organization website also has regularly updated information about outbreaks.
The approach to evaluation of fever in the returning traveler will be reviewed here. Other issues related to travel are discussed separately:
● (See "Travel advice" .)
● (See "Immunizations for travel" .)

Type your tag names separated by a space and hit enter
Fever in the returned traveler from tropical areas
- 21% of all fevers and 59% of undifferentiated fevers.
- The leading cause of travel-related hospitalization and death.
- Commonly seen species include P. falciparum (high case fatality rate if untreated), P. vivax, P. ovale, P. malariae, P. knowlesi (simian malaria found in Southeast Asia, rarely transmissible to humans and potentially fatal).
- Sub-Saharan Africa: 49% with a febrile illness of whom 42% with malaria
- The Americas (Central & South America and the Caribbean): 25% with febrile illness of whom 8% had malaria
- Southeast Asia: 34% with a febrile illness of whom 7% had malaria
- Zika virus often co-circulates with Dengue and Chikungunya viruses but the proportion of febrile illnesses due to this virus is unknown.
- R. africae (cause of tick-bite fever) especially common after safaris or treks in Southern Africa.
- O. tsutsugamushi: scrub typhus (from chiggers), one of the more overlooked causes, especially in Asia.
- Diarrhea/dysentery + fever: non-typhoidal Salmonella spp , Shigella spp., and Campylobacter spp . are among the most commonly isolated organisms; fever is only seen in 10% of patients with E. histolytica (amebic dysentery).
- Salmonella enterica serovar typhi or paratyphi (enteric fever): 2% of all fever and 6% of undifferentiated fever
- Urinary tract infection/ pyelonephritis : 3% of all fevers.
- Tuberculosis : < 1% of all fevers.
- Since 2013, cases reported in the Caribbean region (Saint Martin, Saint Barthelemy, Martinique, Guadeloupe, and Guyana). It may be associated with a fever/undifferentiated fever but is usually associated with arthralgia, which can be severe and may become chronic.
- Dengue and Zika virus infections are often part of ddx.
- Uncommon pathogens in systemic illnesses: also consider leptospirosis , amoebic liver abscess , Q fever , melioidosis , viral meningitis , relapsing fever
- Approximately 30% of persons seeking medical care following travel have a fever. Subsets listed in Table Table 1
- Calculate the Quick Sepsis-related Organ Failure Assessment (qSOFA) [3] or calculate a standard SOFA using online tools ( http://clincalc.com/icumortality/sofa.aspx ) to determine the risk of severe sepsis and the need for urgent empirical treatment.
- Malaria should always be suspected in any febrile person returning from an endemic region until proven otherwise.
- Obtaining detailed geographic travel and exposure history (including modes of possible exposure), vaccination history and treatment history is essential.
- Consider algorithmic thinking based upon the qSOFA score and signs/symptoms accompanying the fever to guide differential diagnostic considerations. [2]
- Malaria for every febrile patient who has been in a malarious area.
- Enteric fever: needs to be considered as both Salmonella typhi and S. paratyphi , types A and B can cause a potentially life-threatening undifferentiated fever without other signs or symptoms.
- Exposure history provides clues to certain pathogens. See Table Table 2 .
- Undifferentiated fever in the returned traveler poses the greatest challenge.
- Specific localizing findings should be used to help guide the clinician’s evaluation of each patient whenever possible. For example, look carefully for rash, lymphadenopathy, and/or hepatosplenomegaly.
- Malaria (consider as leading dx if the person has been in a malarious area since falciparum malaria can be fatal in non-immune persons, and chemoprophylaxis is not 100% protective).
- Enteric fever (typhoid and paratyphoid)
- Spotted fevers and typhus group rickettsiae
- Scrub typhus
- Less common: chikungunya , brucellosis , leptospirosis , acute HIV or STDs, tick- and louse-borne-relapsing fevers, tularemia , and non-tropical disease ( infectious mononucleosis , endocarditis , lymphoma).
- Meningococcemia
- Leptospirosis
- Yellow fever
- Congo-Crimean hemorrhagic fever
- Hemorrhagic fevers of South America (Manchupo, Junin, Sabia, and Guanarito viruses)
- Most are Biosafety Level-4 agents; if suspicious, immediately notify local/state health authorities.
- Survival of VHF viruses in nature is dependent on an animal or insect host; geographically restricted distribution of diseases is based upon the host.
- Humans are not the natural reservoir for any of these viruses. Human cases or outbreaks of VHFs occur sporadically and usually are not easy to predict.
- Meningococcal meningitis and other causes of meningitis
- African trypanosomiasis (sleeping sickness)
- Tick-borne encephalitis (TBEV)
- Japanese encephalitis
- West Nile encephalitis
- Angiostrongyloides cantonensis
- Nipah virus
- Seasonal or avian influenza
- Pneumococcal pneumonia
- Legionellosis
- Q fever (may have a longer incubation period)
- Melioidosis
- Acute histoplasmosis
- Acute coccidioidomycosis
- Hantavirus pulmonary syndrome
- Coronavirus including Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV2)
- Plague (extremely rare in travelers)
- Malaria (leading dx if the person has been in a malarious area).
- Many of the other diseases noted above can have incubation > 2 wks, including many of the hemorrhagic fevers and fungal infections
- Brucellosis
- Typhoid fever
- Sleeping sickness
- Amebic liver abscess
- Hepatitis A or Hepatitis E . Now that hepatitis A and hepatitis B vaccines are childhood immunizations and increasingly hepatitis A vaccine is given to adults prior to travel, hepatitis E is a more important pathogen
- Acute schistosomiasis
- Acute toxoplasmosis
- Bartonellosis
- Malaria (leading dx if the person has been in a malarious area)
- Hepatitis B
- Tuberculosis
- Visceral leishmaniasis
- Fascioliasis
- Many of the infections noted also occur with shorter incubation. Consultation with a tropical medicine/infectious disease expert is recommended.
Chikungunya
- Dengue fever
- Enteric feve r
- Lyme disease
- HIV, acute infection syndrome
- Rickettsial infection
- Schistosomiasis , acute syndrome
- Sub-Saharan Africa : malaria >>> other causes of undifferentiated fever.
- Southeast Asia : dengue > malaria >>> other causes of undifferentiated fever.
- South-Central Asia, especially India : malaria = typhoid/paratyphoid fever (especially if visiting family) = dengue .
- Latin America/Caribbean : dengue > malaria >>> other causes of undifferentiated fever
- See individual modules for additional, detailed information.
- Rapid tests (e.g., histidine-rich protein [HRP]-2 antigen detection): highly specific but not as sensitive as thick and thin smears. But, provide results in about 15 minutes. But, a positive test or negative test does NOT eliminate the need for microscopy.
- P. knowlesi usually infects monkeys and rarely humans in SE Asia; can be mistaken for P. malariae on smear; suspect if high-level parasitemia (≥2.5% of RBCs infected) and lab reports P. malariae. PCR identification is needed to confirm dx.
- Falciparum malaria, 65%
- Vivax malaria typically presents >2 months after return (60%).
- CBC with differential and platelets, blood cultures
- Urinalysis (with culture if abnormal sediment)
- Liver enzymes
- Most (66%) present within 7 d of return.
- Leukopenia +/- thrombocytopenia.
- Severe infection may cause hemorrhage, seizures, and shock.
- Diagnosis: serology (IgM, collected >5 days after symptom onset), virus isolation from blood (research labs only), PCR.
- Culture is the most sensitive and specific method but is restricted to reference laboratories [bio-hazard].
- PCR detection possible at specialized laboratories performed best performed on swabs of inoculation eschar or tissue biopsies.
- Pathogen-specific serology is the most commonly used in dx but often tests available (e.g., Weil-Felix or immunofluorescence assay) cannot distinguish species due to cross-reactivity. Western blot and cross-absorption are available only in reference laboratories.
- Specific rickettsial species based upon geographic location of acquisition.
- Bone marrow culture is 90% sensitive and not affected by up to 5 days of antibiotics, but rarely performed in the US.
- Serologic tests lack sensitivity and specificity.
- Current attenuated and killed typhoid vaccines are only 60-70% effective in preventing S. typhi (only).
- Institute barrier isolation in a private room until communicable agents are ruled out.
- Any returned traveler with hemorrhagic manifestations requires URGENT intervention.
- Obtain an infectious disease consult, inform infection control and contact the Special Pathogens Branch at CDC Division of Viral Diseases in Atlanta Ga (404.639.1511) for assistance.
- Fever with pulmonary findings : viral and bacterial cultures of respiratory secretions, CXR or chest CT.
- Obtain blood cultures.
- Serology (MAT preferred, available at CDC)
- Culture urine, blood, CSF (alert lab, need special Fletcher’s media).
- Liver aspirate (usually not done if serology is available).
- Serology has >95% sensitivity.
- Viral meningitis : lumbar puncture, culture and PCR for arboviruses, enterovirus .
- Virus isolation: possible isolation from acute serum specimens (< 8 days) of illness.
- RT-PCR: usually positive in the first week after symptom onset
- Serology: IgM virus-specific antibody by capture ELISA or a 4-fold rise IgG antibody between acute (during 1st 8 days of illness) and convalescent (10-14 days after the first) antibody.
Uncomplicated Plasmodium falciparum , Plasmodium knowlesi or species unspecified
- "Presumptive treatment" without the benefit of laboratory confirmation should be reserved for extreme circumstances (strong clinical suspicion, severe disease, impossibility of obtaining prompt laboratory confirmation).
- If the patient used malaria chemoprophylaxis, choose a different agent for treatment.
- Note chloroquine-resistant P. falciparum areas in the Middle East including Iran, Oman, Saudi Arabia and Yemen.
- Chloroquine phosphate (Aralen and generics) 600 mg base (1,000 mg salt) orally immediately, followed by 300 mg base (500 mg salt) PO at 6h, 24h and 48h. Total dose: 1,500 mg base (2,500 mg salt).
- Hydroxychloroquine 620 mg base (800 mg salt) PO initially then 310 mg base (400 mg salt) at 6h, 24h and 48h after the initial doses (total 1550 mg = 2000 mg salt).
- Alternatives: any treatment for chloroquine-resistant malaria (noted below).
- Artemether/lumefantrine 20 mg/120 mg (Coartem TM ) 4 tablets orally at hour 0 and hour 8 during the first 24 hours. Then 4 tablets twice daily on days 2 and 3 (total treatment is 24 tablets. Pediatric dosing for 2 months to 16 years is by weight (see Table Table 3 ).
- Quinine sulfate plus one of the following: doxycycline , tetracycline or clindamycin .
- Quinine sulfate: 542 mg base (650 mg salt) PO three times daily x 3 (if malaria acquired outside of Southeast Asia) to 7 days (for malaria acquired in Southeast Asia).
- Doxycycline : 100 mg PO twice daily x 7 days. DO NOT use for treatment if this agent was used for chemoprophylaxis
- Tetracycline 250 mg PO four times daily x 7 days. DO NOT use for treatment if this doxycycline was used for chemoprophylaxis
- Clindamycin 20 mg base/kg/day PO divided in three times daily x 7 days ( clindamycin may be less efficacious than doxycycline or tetracycline when combined with quinine ).
- Least preferred treatment : mefloquine (generics only) 684 mg base (750 mg salt) PO as initial dose, followed by 456 mg base (500 mg salt) PO given 6-12 hours after initial dose. Total dose = 1,250 mg salt. DO NOT use for treatment if this agent was used for chemoprophylaxis
Uncomplicated Plasmodium malariae (all regions of the world)
- Preferred: chloroquine phosphate (Aralen and generics) 600 mg base (1,000 mg salt) oral immediately followed by 300 mg base (500 mg salt) PO at 6h, 24h and 48h. Total dose: 1,500 mg base (2,500 mg salt).
- Alternative: hydroxychloroquine (Plaquenil TM and generics) 620 mg base (800 mg salt) PO immediately, followed by 310 mg base (400 mg salt) PO at 6h, 24h and 48h. Total dose: 1,550 mg base (2,000 mg salt).
Uncomplicated Plasmodium vivax and P. ovale
- All P. ovale is sensitive to chloroquine.
- All P. vivax is sensitive to chloroquine except that acquired in Papua New Guinea or Indonesia. Although isolated cases of P. vivax have been reported from other areas of the world, initial treatment should be with chloroquine.
- Chloroquine phosphate 1g salt (600 mg base) orally once, then 500 mg salt (300 mg base) 6 h later, then 500 mg at 24 h and 48 h followed by primaquine phosphate PO x 14 days (see below)
- Hydroxychloroquine as above.
- Adult dosing 30 mg base PO daily x 14 days
- Pediatric dosing: 0.5 mg/kg once daily for 14 days not to exceed a maximum dose: 30 mg/day.
- Adult dosing: 300 mg orally once
- Pediatric dosing (for patients 16 years and older): 300 mg orally once
- Consult an infectious or tropical diseases specialist for the treatment of patients with G6PD deficiency or with decreased G6PD activity.
- Pediatric dosing: single per weight dose per day x 4 days as in Table Table 4 above.
- Quinine sulfate plus either doxycycline or tetracycline (contraindicated in children < 8 years) followed by primaquine phosphate as above
- Children < 8 years option: mefloquine 15 mg/kg followed 12 hours later by 10 mg/kg/dose followed by primaquine phosphate as outlined above.
- If clinical improvement is not seen within 48-72 hours, alternative therapy should be used for retreatment.
- Artemether/lumefantrine 20 mg/120 mg (Coartem TM ) 4 tablets orally at hour 0 and hour 8 during the first 24 hours. Then 4 tablets twice daily on days 2 and 3 (total treatment is 24 tablets. Pediatric dosing for 2 months to 16 years is by weight (See Table Table 3 ). Persons with P.vivax or P. ovale should receive primaquine to prevent relapse ( 0.5 mg/kg orally once daily for 14 days (maximum dose = 30 mg base).
- Areas where chloroquine-resistant P. falciparum co-circulates with P. vivax: consider treatment with one of the oral treatments outline for uncomplicated P. falciparum provided in previous sections.
Uncomplicated Malaria, Pregnant Women
- Primaquine phosphate is contraindicated in pregnancy and breastfeeding women and should not be used at the end of treatment for the liver stage of P. vivax or P. ovale .
- Following treatment for P. vivax or P. ovale, the pregnant patient should be maintained on chloroquine or hydroxychloroquine at chemoprophylactic doses for the remainder of the pregnancy and then women without G6PD deficiency should be receive treated with primaquine phosphate following delivery.
- If breastfeeding is planned, the chemoprophylactic regimen should be maintained throughout breastfeeding and final primaquine treatment provided at the end of breastfeeding.
- Quinine sulfate: 542 mg base (650 mg salt) PO three times daily x 3 to 7 days AND clindamycin : 20 mg base/kg/day PO divided three times daily x 7 days.
- Southeast Asia = 7 days
- Infections acquired elsewhere = 3 days
- Quinine sulfate 650 mg salt PO three times daily x 7 days.
- Thereafter, the woman should be maintained on weekly mefloquine at chemoprophylactic dosing for the remainder of pregnancy as mefloquine is FDA approved for chemoprophylaxis in pregnancy.
- Women without G6PD deficiency should receive primaquine phosphate following delivery. If breastfeeding is planned, the chemoprophylactic regimen should be maintained throughout breastfeeding and final primaquine treatment provided at the end of breastfeeding.
- Primaquine is contraindicated in pregnant and lactating women.
- Mefloquine is generally not recommended for treatment in pregnant women due to a possible increase in stillbirths; however, it may be used if it is the only treatment option available and if the potential benefit is judged to outweigh the potential risks. Mefloquine is approved for use in pregnancy for chemoprophylaxis.
- Doxycycline and tetracycline are not generally used in pregnant women. However, one or the other may be used in combination with quinine if other treatment options are not available or not tolerated.
- Atovaquone/proguanil (Malarone TM ) and artemether/lumefantrine (Coartem TM ) are classified as pregnancy Category C agents, not usually recommended. However, if other treatment options are not available or not tolerated, agents can be considered. After delivery, women without G6PD deficiency should be treated with primaquine phosphate as previously outlined.
Complicated/Severe Plasmodium falciparum or intolerance of oral drugs (all regions)
- Oral treatment is NOT recommended for the treatment of severe/complicated malaria.
- 1 dose = 2.4 mg/kg
- IV doses (3 in total) at 0, 12, and 24 hours
- NOTA BENE: If IV artesunate is not readily available: INITIALLY give ORAL antimalarials (see the above) while obtaining IV Artesunate. When IV artesunate arrives, discontinue oral antimalaria and initiate IV treatment. IF ORAL TREATMENT is NOT tolerated, consider administration by nasogastric tube or give after and antiemetic.
- PLUS reassessment and follow-on treatment below
- Reassess parasite density at least 4 hours after the 3rd IV dose of artesunate
- Artemether-lumefantrine (CoArtem TM ): PREFERRED; or
- Atovoquone-proguanil (Malarone TM ); or
- Quinine sulfate PLUS doxycycline OR in children less than 8 years or pregnant women substitute doxycycline with clindamycin; or
- Mefloquine (only if NO OTHER TREATMENT OPTIONS ARE AVAILABLE)
- Able to take oral medications: Give the complete follow-on regimen (See Uncomplicated falciparum malaria above for oral dosing)
- If unable to take oral medications, continue IV artesunate every day for up to 6 more days until the patient can take oral medication and then give the complete follow-on regimen
- IV artesunate is well tolerated.
- While rare, delayed post-artemisinin hemolytic anemia has been noted in published case reports following treatment of severe malaria with IV artesunate.
- Persons with higher parasite density seem to have a higher likelihood of delayed hemolytic anemia after treatment.
- Hemoglobin,
- Reticulocyte count,
- Haptoglobin,
- Lactate dehydrogenase (LDH), and
- Total bilirubin.
- Depending on the intensity of hemolysis and the presence of anemia signs and symptoms, a blood transfusion may be needed.
- Those receiving IV artesunate from CDC should be reported to CDC no longer than 24 hours after diagnosis.
- Those receiving commercially available Artesunate for Injection TM should be reported to MedWatch, FDA’s Safety Information and Adverse Event Reporting Program.
- Exchange transfusion: no longer considered routine recommendation as this procedure has not been proven to be beneficial in an adequately powered randomized clinical trial.
Febrile Diarrhea/Dysentery
- Antibiotic resistance patterns (see above) should guide treatment or modification of empiric therapy.
- Ceftriaxone 1-2 g IV once daily
- Cefotaxime 2 g IV every 8 h
- Change to other agents based on resistance pattern.
- All immunocompromised persons (organ transplant, HIV-infected, persons who received corticosteroids or immunosuppressive therapies), those with sickle cell disease, hemoglobinopathies, cirrhosis, cancer or lymphoproliferative disease: treat regardless of the severity of symptoms.
- Antibiotic resistance is widespread and increasing. High levels of multidrug resistance (ciprofloxacin, trimethoprim-sulfamethoxazole, and azithromycin) in the Asian Subcontinent and Sub-Sahran Africa)
- Age >64 years, malnourished persons, all HIV-infected persons regardless of CD4 count or viral load, bacteremic persons, food handlers.
- Levofloxacin : 500 mg orally once daily x 3 d
- Ciprofloxacin 500 mg orally twice daily x 5 d
- Azithromycin : 500 mg orally once daily x 3 days
- Cefixime : 200 mg orally twice daily for 5 days
- Trimethoprim-sulfamethoxazole 160/800 mg (one double-strength tablet) orally twice daily x 5 days
- Ampicillin : 500 mg orally every 6 hours x 5 days
- Base final treatment on culture and sensitivity results (consider adding 2-4 days of treatment for immunocompromised patients)
- Drug resistance is widespread and increasing and is higher for fluoroquinolones compared with macrolides.
- Preferred: azithromycin 500 mg orally every day x 3 days or until signs and symptoms of de
- Alternative erythromycin stearate 500 mg orally twice daily for 5 days
- Adults: 500-750 mg orally three times daily x 10 d
- Children: 35 - 50 mg/kg/day in 3 divided doses x 7-10 d
- Adults:2 grams orally x 3 d
- Adults: 500 mg orally daily x 3 d
- Children: 40 mg/kg/d orally x 3 d
- Adults: 2 grams orally daily x 1-3 d
- Children: 30 mg/kg/d orally x 1-3 d
- Mild to moderate disease: Paromomycin 25 - 35 mg/kg/d in 3 divided doses x 5-10 d
- Severe disease: Metronidazole as above
- Iodoquinol 650 mg orally three times daily for 20 d
- Paromomycin 25-35 mg/kg/d orally divided three times daily for 7 d
- Diloxanide (not available in the U.S.) 500 mg orally three times daily for 10 d
- Fulminant cases should include treatment of gram-negative organisms. In the uncommon setting of toxic megacolon, surgical intervention may be needed.
Enteric Fever (Salmonella enterica serovar typhi or paratyphi)
- Ceftriaxone : 1-2 g IV once daily for 7-14 d
- Alternate if ceftriaxone is not available: Cefotaxime : 1-2 g IV every 8 h for 7-14 d
- Meropenem : 1 -2 g IV every 8 h x 7-14 d
- May substitute other carbapenems if meropenem is not available.
- Ciprofloxacin 250-500 mg orally twice daily for 7-14d
- Cefixime : 200 mg orally twice daily x 10-14 d
- Azithromycin : 1 g orally then 500 mg orally daily OR 1 g orally once daily x 5-7 d
- Cefixime : 20 mg/kg orally in 2 divided doses (maximum 400 mg per day) x 10-14 d
- Azithromycin : 10 - 20 mg/kg orally conce per day (maximum 1000 mg/d) x 5-7 d
- Ciprofloxacin 500 mg IV every 12 h daily for 10-14d
- Ceftriaxone : 2 g IV once or twice daily x 10-14 d
- Cefotaxime : 150-200 mg/kg IV/d in 3-4 divided doses (maximum 8 g/d) x 10-14 d
- Azithromycin : 10 - 20 mg/kg orally once per day (maximum 1000 mg/d) x 5-7 d
- Ciprofloxacin (justified use in severe infection): 20 mg/kg/d in 2 divided doses (maximum 800 mg/d) x 7-10 d
- Ceftriaxone : 50-100 mg/kg IV in 1 or 2 divided doses (maximum 4 g/d) x 10-14 d
- Cefotaxime : 150-200 mg/kg IV in 3-4 divided doses (maximum 8 g/d) x 10-14
- Dexamethasone: use controversial, may decrease mortality in severe typhoid fever cases where delirium, coma, obtundation or stupor are present.
- An infectious disease specialist should be consulted in all cases of typhoid fever given its low prevalence in the developed world.
- Consult a surgeon if GI perforation, GI hemorrhage is suspect.
- Ileal perforation usually occurs in the third week of febrile illness.
- Relapse (typhoid fever): occurs in 1-6% of immunocompetent persons, typically 2-3 weeks following resolution of symptoms.
- No chikungunya -specific antiviral drug treatment is available.
- Avoid aspirin because of bleeding in a small number of patients and the risk of Reye’s syndrome in children younger than 12 years of age.
- Acetaminophen or NSAIDs may help to relieve the arthritic component of the disease.
- Fluid repletion important
Rickettsial infections
- If suspected, begin treatment empirically since diagnostic test results are often delayed or not performed.
- Outpatient: doxycycline 100 mg orally twice daily x 5 d or until 48h after defervescence.
- Consider doxycycline 200mg loading dose.
- Pregnant women: if a life-threatening infection, doxycycline should be used despite being a Category D agent. Consider consultation with an infectious disease expert.
Urinary Tract Infections/pyelonephritis
- Acute bacterial cystitis: see module .
- Pyelonephritis: see module .
- Urinary tract infections in pregnant women: see module .
- Usually a self-limited disease; requires only supportive treatment.
- Avoid NSAIDs, aspirin, and steroids.
- Closely monitor all patients with evidence of hemorrhagic-associated symptoms: tachycardia, prolonged capillary refill time, cool or mottled skin, evidence of volume depletion, narrowed pulse pressure, hypotension, rising packed cell volume or falling platelet count. Such patients should be hospitalized for correction of volume deficits and for monitoring.
- If dengue hemorrhagic fever is suspected, consider consulting with a specialist.
- Usually is a self-limited disease requiring only supportive measures.
- Avoid NSAIDs, aspirin, and steroids
- Pregnant women need to be assessed and monitored by a maternal-fetal expert.
- Sexual transmission to all partners is possible, and therefore either abstaining from sex or using latex condoms/dental dams for every mucosally lined orifice contact for at least 2 months with partners of infected women and at least 6 months with partners of infected men is recommended.
- A risk of Guillain Barre syndrome is present for weeks following infection although the proportionate risk is small.
TREATMENT REGIMEN DETAILS
Severe Malaria:
- Impaired consciousness/coma
- Severe normocytic anemia
- Renal failure
- Pulmonary edema
- Acute respiratory distress syndrome
- Circulatory shock
- Disseminated intravascular coagulation
- Spontaneous bleeding
- Hemoglobinuria
- Repeated generalized convulsions
- Parasitemia of >5%
- Treatment with IV quinidine should be initiated as soon as possible after diagnosis is secured. Use IV loading dose of quinidine unless they have received more than 40 mg/kg of quinine in the preceding 48 hours or if they have received mefloquine within the preceding 12 hours.
- Consultation with a cardiologist and a physician with experience treating malaria (if available) is advised when treating malaria with quinidine .
- Blood pressure monitoring (hypotension)
- Cardiac monitoring (widened QRS complex and/or lengthened QTc interval)
- Blood glucose checks (hypoglycemia)
- Cardiac complications: if severe may warrant temporary discontinuation of the drug or slowing of the intravenous infusion. Do NOT delay treatment with quinidine while waiting for parenteral artesunate (to arrive from CDC, for example).
- See Treatment of complicated/severe P. falciparum section for use of investigational artemisinin for patients with severe malaria.
- Exchange transfusion if the parasite density (i.e. parasitemia) is >10% remains less favored now than historically [17] OR if the patient has altered mental status, non-volume overload pulmonary edema, or renal complications. Since 2013, CDC does not recommend red blood cell exchange transfusion.
- If exchange transfusion is employed, continue until the parasite density is < 1% (usually requires 8-10 units).
- IV quinidine administration should not be delayed for exchange transfusion and can be given concurrently throughout the exchange transfusion.
- Pregnant women diagnosed with severe malaria should be treated aggressively with parenteral antimalarial therapy.
Selected Drug Comments
Other information.
- Consider consultation with an infectious disease- or tropical medicine expert for any returned traveler with undifferentiated fever, especially if suspects include malaria , enteric fever, or viral hemorrhagic fever, or the patient has neurological findings.
- Malaria in pregnancy is associated with high rates of both maternal and perinatal morbidity and mortality. Pregnant women are three times more likely to develop severe disease than non-pregnant women acquiring infection in the same area. Infection in pregnancy can lead to spontaneous abortion, premature delivery, low birth weight, congenital infection, and perinatal death.
Basis for recommendation
Comment: ABSTRACT: Malaria infection during pregnancy is associated with an increased risk for maternal and fetal complications. In the United States, treatment options for uncomplicated, chloroquine-resistant Plasmodium falciparum and P. vivax malaria in pregnant women are limited to mefloquine or quinine plus clindamycin (1). However, the limited availability of quinine and increasing resistance to mefloquine restrict these options. Strong evidence now demonstrates that artemether-lumefantrine (AL) (Coartem) is effective and safe in the treatment of malaria in pregnancy. The World Health Organization (WHO) has endorsed artemisinin-based combination therapies (ACTs), such as AL, for the treatment of uncomplicated malaria during the second and third trimesters of pregnancy and is currently considering whether to add ACTs, including AL, as an option for malaria treatment during the first trimester (2,3). This policy note reviews the evidence and updates CDC recommendations to include AL as a treatment option for uncomplicated malaria during the second and third trimesters of pregnancy and during the first trimester of pregnancy when other treatment options are unavailable. These updated recommendations reflect current evidence and are consistent with WHO treatment guidelines.
Comment: This is an outstanding review. It provides a useful algorithm to guide the workup of the febrile returning traveler, diagnoses for consideration. Additionally, there are 3 concise tables that would be of great use to the clinician. The first addresses life-threatening conditions (viral, bacterial and protozoal) which should not be missed. The second concisely outlines the critical areas in the travel and medical history to be considered in assessing the febrile returned traveler and the diseases to consider when risks are identified. The third table has both clinical and public health implications as it concisely summarizes the serious transmissible infections.
Comment: This is an excellent editorial that summarizes the pros and cons of the use of the qSOFA vs the standard SOFA. The ability to rapidly apply the qSOFA to guide intervention at the time of presentation to an emergency department without relying on formulae or websites should be considered in assessing this tool’s usefulness. The qSOFA score ranges from 0 to 3. One point is given for each of the following: SBP < /=100 mm Hg; respiratory rate >/= 22/min, and evidence of altered mental status on Glasgow Coma Score (normal = 15, range 3-15).
Comment: The malaria treatment guidelines are provided online for clinicians and updated as needed by CDC. IMPORTANT: All persons treated for severe malaria with IV artesunate should be monitored weekly for up to four weeks after treatment initiation for evidence of hemolytic anemia.
Comment: This alphavirus carried by Aedes spp mosquitos has re-emerged in recent years in Africa, southern and SE Asia and the Indian Ocean islands (and now has appeared for the first time in the Caribbean islands). The disease associated with infection is usually associated with fever, headache, myalgia, rash and arthralgia which can be acute as well as chronic. It traditionally was thought to be associated with varying morbidity but only since 2005 has mortality been noted. Because Aedes spp, particularly Ae. albopictus is common in both Europe and the U.S. and infected larvae can overwinter, this virus poses a threat in the Americas. Rating: Important
Comment: This is an analysis of data collected in the GeoSentinel Surveillance system examining the risk of illnesses amongst 4779 ill travelers to common destinations in Mexico and Central America in the period 1996 to 2010. Although malaria was not commonly diagnosed at participating surveillance sites when compared with travelers to subSaharan Africa or parts of Asia, malaria was seen increasingly with more southern travel. The most frequent presenting syndromes included acute and chronic diarrhea, dermatologic diseases, febrile systemic illness, and respiratory disease. The overall risk of malaria was low; only 4 cases of malaria were acquired in Mexico (Proportionate Morbidity [PM} of 2.0 per1000 ill returned travelers) in 13 years, compared with 18 from Honduras (PM, 79.6 cases per 1000 ill returned travelers) and 14 from Guatemala (PM, 34.4 cases per 1000 ill returned travelers) during the same period. Plasmodium vivax malaria was the most frequent malaria diagnosis.
Comment: This is an analysis of 2004-2008 Salmonella spp isolates submitted to the CDC’s FoodNet foodborne disease active surveillance network in which travel-acquired infections were compared with non-travel associated infections. Among 23,712 reported cases with known travel status, 11% had traveled internationally in the 7 days before illness. Travelers with Salmonella infection tended to be older (median age, 30 years) than non-travelers (median age, 24 years; p< 0.0001), but were similar with respect to gender. The most common destinations reported were Mexico (38% of travel-associated infections), India (9%), Jamaica (7%), the Dominican Republic (4%), China (3%), and the Bahamas (2%). The 2 most commonly reported serotypes, regardless of travel status, were Enteritidis (19% of cases), and Typhimurium (14%). However, serotypes associated with enteric fever (S. typhi and S. paratyphi) were found in 10% of samples from travelers but only 0.5% of samples submitted from non-travelers.
Comment: Examines the increase in dengue worldwide as well as the reintroduction of endemic foci in the U.S. in southern Texas and in Key West and mainland Florida.
Comment: This prospective study of 1,091 adult patients with proven severe malaria (per WHO criteria) admitted to multiple hospital medical services affiliated with a single Indian medical school from September 2003 through December 2005. Severe monoinfection P. vivax was defined as severe malaria by WHO criteria, peripheral blood smear (PBS), rapid diagnostic test (RDT) and polymerase chain reaction (PCR) positive for P. vivax and negative for P. falciparum. Of 1,091 patients with malaria, 635 had P. falciparum malaria and 456 had P. vivax malaria; 40 had evidence of monoinfection of P. vivax; age 18-62 years with a mean of 30 years; most were male. Complications observed among this group were hepatic dysfunction and jaundice in 23 (57.5%) patients, renal failure in 18 (45%) patients, severe anemia in 13 (32.5%) patients, cerebral malaria in 5 patients (12.5%), acute respiratory distress syndrome in 4 patients (10%), shock in 3 patients (7.5%), and hypoglycemia in 1 (2.5%) patient, 2 (5%) died. Rating: Important
Comment: This case series describes 62 (60% males) consecutive adult patients presenting for care after returning to France from overseas with fever (>38 o C) and exanthema (widespread rash) between January 2006 and September 2007. The most common diagnoses included chikungunya (35%), dengue (26%), and African tick-bite fever (10%). The cause of the rash was not identified in 8%. Other causes accounting for 1-5% of illnesses were: infectious mononucleosis, primary HIV infection, DMV, measles, rubella, varicella, primary toxoplasmosis, and acute schistosomiasis. When comparing chikungunya with dengue virus infection those with dengue infection had significantly greater leukopenia, neutropenia, lymphopenia, thrombocytopenia, and headache. Notably, those with chikungunya had characteristic arthralgia (100%) whereas arthralgia was absent in those with dengue infection.
Comment: This was a retrospective laboratory-based study of 960 filter paper blood spots collected from slide-positive malaria diagnosed amongst hospitalized persons by the Malaysian Ministry of Health between 2001 and 2006. Diagnostic microscopy recorded 44.6% P. vivax ; 32.5% P. malariae , 22.5% P. falciparum , 0.2% P. ovale , and 0.2% mixed infections. P. knowlesi was detected in 260 of 960 (27.7%) of these samples by nested PCR; only 4 (0.4%) were confirmed as P. malariae . Additionally, 54 archived slides from 2003-2005 from outlying district hospitals and clinics with microscopically diagnosed P. malariae were further evaluated after whole blood slide extraction and nested PCR. 46 (85%) were found to be P. knowlesi ; 5(15%) were confirmed as P. malariae . Four of the 46 archival cases were fatal; all had high parasitemia and significant hepato-renal dysfunction. These data suggest that P. knowlesi is not as rare as previously thought and suggest that aggressive management similar to that given for P. falciparum is warranted given the observed case fatality. Rating: Important
Comment: This prospective study of 118 febrile (T ≥37.5 o C by axilla) travelers >14 years of age, 10 days before or 10 days after their return without a specific diagnosis made on their first clinic visit upon return to Spain. All had nasopharyngeal swabs, blood, stool cultures collected. Malaria was sought in all patients. Amongst the group 73 had only respiratory symptoms, 12 had gastrointestinal (GI) symptoms, 5 had both respiratory and GI symptoms, and 28 had an undifferentiated febrile illness. In the respiratory and respiratory/GI illness group 44 were found to have a viral or bacterial respiratory pathogen with 46% of travelers to Latin America infected, and 37% of travelers to both Asia and Africa. The influenza virus was isolated from 18 persons (12 influenza A and 6 influenza B). Influenza A virus was isolated from travelers returning from Asia > Africa and Latin America. Rhinovirus was isolated from 11 persons returning from all continents. Parainfluenza viruses were isolated from 6 travelers and RSV and adenovirus from 4 each.
Comment: This is a report from the GeoSentinel Surveillance Network of 24,920 returning ill travelers evaluated in 31 international sites over a 10-year period ending in 2006. Febrile illness was the chief complaint in 6,957 persons (28%) seeking care post-travel; 26% of this group were hospitalized versus 3% of afebrile travelers. Amongst febrile persons, 35% had an undifferentiated fever, 15% had febrile diarrhea, and 14% had a febrile respiratory illness. The etiology of fever was dependent upon the region of the world that was visited and the reason for visit. Malaria was the most common specific diagnosis identified (21%) and accounted for 4 of 12 deaths among febrile travelers. The next most common cause of fever was dengue followed by enteric fever and rickettsial diseases. Vaccine-preventable infections were seen in 3% of travelers with fever ( S. typhi infection [n=100], acute hepatitis A [n=41], and influenza A [n=29]. Those traveling to visit friends and relatives, so-called VFR travelers were more likely to have a vaccine-preventable febrile illness when compared to other febrile travelers (Odds Ratio 1.8, confidence interval 1.4-2.4, p< 0.001). Rating: Important
Comment: This is an analysis of data collected from 30 sites participating in the CDC-sponsored GeoSentinel Surveillance Network of specialized travel or tropical medicine clinics on 6 continents regarding 17,353 ill returned travelers (persons who had crossed an international border in the 10 years prior to presenting with their illness). Significant differences were noted in proportionate traveler morbidity and mortality among the developing regions of the world. The systemic febrile illness (SFI) rate was 594 per 1000 persons with SFI and was the highest in sub-Saharan Africa (718/1000). Malaria accounted for the greatest proportionate morbidity (352/1000 with sub-Saharan Africa accounting for 2-4 times the burden as other regions (622/1000). Dengue was the second leading cause of SFI (352/1000) with the greatest burden seen in travelers returning from Asia and the Caribbean. Acute diarrheal illness was most prevalent among travelers returning from south-central Asia whereas dermatological problems were most frequent in those who had visited the Caribbean, Central or South America. Rating: Important
Comment: This is a literature-based review and additional case synthesis by one world expert. There are 15 recognized tick-borne rickettsioses; 8 of the 15 have been reported in international travelers (African tick-bite fever, Mediterranean spotted fever, Indian tick typhus, Astrakhan fever, Rocky Mountain spotted fever, Queensland tick typhus, R. aeschlimannii infection and North Asian tick typhus. Off the ~400 cases of tick-borne rickettsioses reported among international travelers, most are due to either Rickettsia africae (Subsaharan Africa-African tick-bite fever) or R. conorii (North Africa/Mid-East/India--Mediterranean spotted fever). The incidence among travelers appears to be increasing for several possible reasons: increased ecotourism increased travel to previously restricted areas (such as to post-apartheid game parks in the Republic of South Africa), and increased diagnostic awareness. Provides recommendations for treatment.
Comment: Mefloquine/artesunate is more effective in the treatment of multi-drug resistant malaria in Thailand than quinine and was found to be safe in pregnancy.
Comment: CDC no longer recommends exchange transfusion for severe malaria due to limited evidence of any efficacy and potential adverse reactions.
Comment: This is the report of a retrospective case review of 708 febrile returning travelers all of whom were tested for Q fever (Coxiella burnetti infection). Five (0.7%) persons were found to be infected. All patients had a fever, 4/5 had a headache, 3/5 had arthralgia and myalgia, one had a dry cough, one was jaundiced, and one complained of malaise. Chest radiographs were normal in all 5, all 5 had an enlarged liver, spleen or both. All initially had normal white blood cell counts in the setting of thrombocytopenia (13,000-98,000 cells/mL) and abnormal transaminases. Treatment was with either doxycycline or ciprofloxacin. All recovered with no complications.
1. Download the Johns Hopkins Guides app by Unbound Medicine
2. Select Try/Buy and follow instructions to begin your free 30-day trial
Want to regain access to Johns Hopkins Guides?
Renew my subscription
Not now - I'd like more time to decide
Log in to Johns Hopkins Guides
Forgot your password, forgot your username, contact support.
- unboundmedicine.com/support
- [email protected]
- 610-627-9090 (Monday - Friday, 9 AM - 5 PM EST.)
Website maintenance is scheduled for Saturday, August 17, and Sunday, August 18. Short disruptions may occur during these days.

NICHOLAS A. RATHJEN, DO, AND S. DAVID SHAHBODAGHI, MD, MPH
Am Fam Physician. 2023;108(4):396-403
Author disclosure: No relevant financial relationships.
Approximately 1.8 billion people will cross an international border by 2030, and 66% of travelers will develop a travel-related illness. Most travel-related illnesses are self-limiting and do not require significant intervention; others could cause significant morbidity or mortality. Physicians should begin with a thorough history and clinical examination to have the highest probability of making the correct diagnosis. Targeted questioning should focus on the type of trip taken, the travel itinerary, and a list of all geographic locations visited. Inquiries should also be made about pretravel preparations, such as chemoprophylactic medications, vaccinations, and any personal protective measures such as insect repellents or specialized clothing. Travelers visiting friends and relatives are at a higher risk of travel-related illnesses and more severe infections. The two most common vaccine-preventable illnesses in travelers are influenza and hepatitis A. Most travel-related illnesses become apparent soon after arriving at home because incubation periods are rarely longer than four to six weeks. The most common illnesses in travelers from resource-rich to resource-poor locations are travelers diarrhea and respiratory infections. Localizing symptoms such as fever with respiratory, gastrointestinal, or skin-related concerns may aid in identifying the underlying etiology.
Globally, it is estimated that 1.8 billion people will cross an international border by 2030. 1 Although Europe is the most common destination, tourism is increasing in developing regions of Asia, Africa, and Latin America. 2 Less than one-half of U.S. travelers seek pretravel medical advice. It is estimated that two-thirds of travelers will develop a travel-related illness; therefore, the ill returning traveler is not uncommon in primary care. 3 Although most of these illnesses are minor and relatively insignificant clinically, the potential exists for serious illness. The advent of modern and interconnected travel networks means that a rare illness or nonendemic infectious disease is never more than 24 hours away. 4 Travelers over the past 10 years have contributed to the increase of emerging infectious diseases such as chikungunya, Zika virus infection, COVID-19, mpox (monkeypox), and Ebola disease. 3
Although most travel-related illnesses are self-limiting and do not require medical evaluation, others could be life-threatening. 5 The challenge for the busy physician is successfully differentiating between the two. Physicians should begin with a thorough history and clinical examination to have the highest probability of making the correct diagnosis. Travelers at the highest risk are those visiting friends and relatives who stay in a country for more than 28 days or travel to Africa. Most travel-related illnesses become apparent soon after arriving home because incubation periods are rarely longer than four to six weeks. 3 , 6 The most common illnesses in travelers from resource-rich to resource-poor locations are travelers diarrhea and respiratory infections. 7 , 8 The incubation period of an illness relative to the onset of symptoms and the length of stay in the foreign destination can exclude infections in the differential diagnosis ( eTable A ) .
General questions should determine the patient’s pertinent medical history, focusing on any unique factors, such as immunocompromising illnesses or underlying risk factors for a travel-related medical concern. Targeted questioning should focus on the type of trip taken and the travel itinerary that includes accommodations, recreational activities, and a list of all geographic locations visited ( Table 1 3 , 6 , 9 and Table 2 3 , 6 ) . Patients should be asked about any medical treatments received in a foreign country. Modern travel itineraries often require multiple stopovers, and it is not uncommon for the casual traveler to visit several locations with different geographically linked illness patterns in a single trip abroad.
Travel History
Travelers visiting friends and relatives are at a higher risk of travel-related illnesses and more severe infections. 10 , 11 These travelers rarely seek pretravel consultation, are less likely to take chemoprophylaxis, and engage in more risky travel-related behaviors such as consuming food from local sources and traveling to more remote locations. 3 Overall, travelers visiting friends and relatives tend to have extended travel stays and are more likely to reside in non–climate-controlled dwellings.
During the clinical history, inquiries should be made about pretravel preparations, including chemoprophylactic medications, vaccinations, and personal protective measures such as insect repellents or specialized clothing. 12 , 13 Accurate knowledge of previous preventive strategies allows for appropriate risk stratification by physicians. Even when used thoroughly, these measures decrease the likelihood of certain illnesses but do not exclude them. 6 Adherence to dietary precautions and pretravel immunization against typhoid fever do not necessarily eliminate the risk of disease. Travelers often have no control over meals prepared in foreign food establishments, and the currently available typhoid vaccines are 60% to 80% effective. 14 Although all travel-related vaccines are important, the two most common vaccine-preventable illnesses in travelers are influenza and hepatitis A. 12 , 15
Travel duration is also an important but often overlooked component of the clinical history because the likelihood of illness increases directly with the length of stay abroad. The longer travelers stay in a non-native environment, the more likely they are to forego travel precautions and adherence to chemoprophylaxis. 3 The use of personal protective measures decreases gradually with the total amount of time in the host environment. 3 A thorough medical and sexual history should be obtained because data show that sexual contact during travel is common and often occurs without the use of barrier contraception. 16
Clinical Assessment
The severity of the illness helps determine if the patient should be admitted to the hospital while the evaluation is in progress. 3 Patients with high fevers, hemorrhagic symptoms, or abnormal laboratory findings should be hospitalized or placed in isolation ( Figure 1 ) . For patients with a higher severity of illness, consultation with an infectious disease or tropical/travel medicine physician is advised. 3 Patients with symptoms that suggest acute malaria (e.g., fever, altered mental status, chills, headaches, myalgias, malaise) should be admitted for observation while the evaluation is expeditiously completed. 13
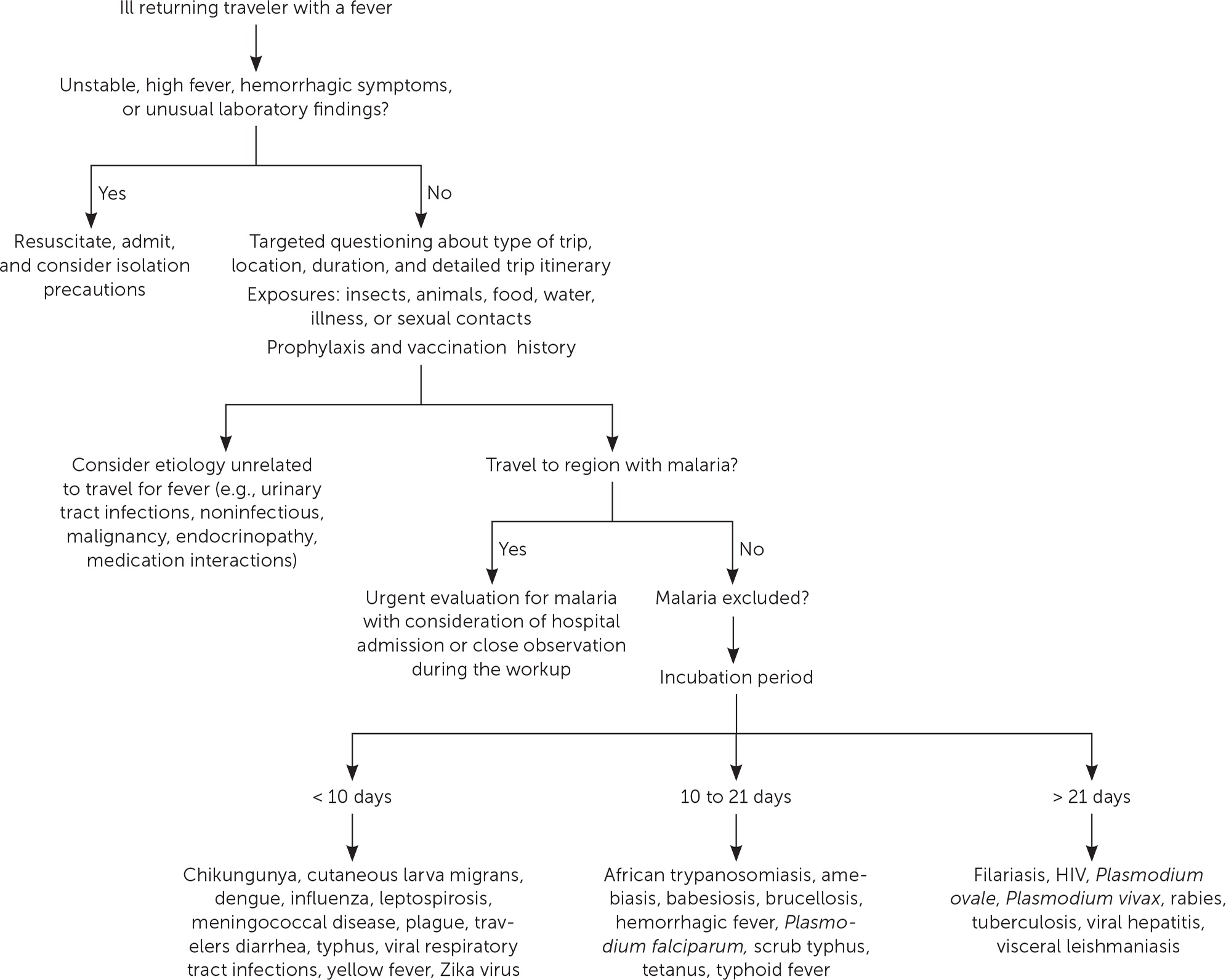
Many tools can assist physicians in making an accurate diagnosis. The GeoSentinel is a worldwide data collection network for the surveillance and research of travel-related illnesses; however, this service requires a subscription. The network can guide physicians to the most likely illness based on geographic location and top diagnoses by geography. 4 For example, Plasmodium falciparum malaria is the most common serious febrile illness in travelers to sub-Saharan Africa. 17
Ill returning travelers should have a laboratory evaluation performed with a complete blood count, comprehensive metabolic panel, and C-reactive protein. Additional testing may include blood-based rapid molecular assays for malaria and arboviruses; blood, stool, and urine cultures; and thick and thin blood smears for malaria. 3 Emerging polymerase chain reaction technologies are becoming widely available across the United States. Multiplex and biofilm array polymerase chain reaction platforms for bacterial, viral, and protozoal pathogens are now available at most tertiary health care centers. 4 Multiplex and biofilm platforms include dedicated panels for respiratory and gastrointestinal illnesses and bloodborne pathogens. These tests allow for real-time or near real-time diagnosis of agents that were previously difficult to isolate outside of the reference laboratory setting.
Table 3 lists common tropical diseases and associated vectors. 3 , 6 , 18 Physicians should be aware of unique and emerging infections, such as viral hemorrhagic fevers, COVID-19, and novel respiratory pathogens, in addition to common illnesses. Testing for infections of public health importance can be performed with assistance from local public health authorities. 19 In cases of short-term travel, previously acquired non–travel-related conditions should be on any list of applicable differential diagnoses. References on infectious diseases endemic in many geographic locations are accessible online. The Centers for Disease Control and Prevention (CDC) Travelers’ Health website provides free resources for patients and health care professionals at https://www.cdc.gov/travel .
Febrile Illness
A fever typically accompanies serious illnesses in returning travelers. Patients with a fever should be treated as moderately ill. One barrier to an accurate and early diagnosis of travel-related infections is the nonspecific nature of the initial symptoms of illness. Often, these symptoms are vague and nonfocal. A febrile illness with a fever as the primary presenting symptom could represent a viral upper respiratory tract infection, acute influenza, or even malaria, typhoid, or dengue, which are the most life-threatening. According to GeoSentinel data, 91% of ill returning travelers with an acute, life-threatening illness present with a fever. 20 All travelers who are febrile and have recently returned from a malarious area should be urgently evaluated for the disease. 13 , 21 Travelers who have symptoms of malaria should seek medical attention, regardless of whether prophylaxis or preventive measures were used. Suspicion of P. falciparum malaria is a medical emergency. 13 Clinical deterioration or death can occur in a malaria-naive patient within 24 to 36 hours. 22 Dengue is an important cause of fever in travelers returning from tropical locations. An estimated 50 million to 100 million global cases of dengue are reported annually, with many more going undetected. 23 eTable B lists the most common causes of fever in the returning traveler.
Respiratory Illness
Respiratory infections are common in the United States and throughout the world. Ill returning travelers with respiratory concerns are statistically most likely to have a viral respiratory tract infection. 24 Influenza circulates year-round in tropical climates and is one of the most common vaccine-preventable illnesses in travelers. 3 , 12 Influenza A and B frequently present with a low-grade fever, cough, congestion, myalgia, and malaise. eTable C lists the most common causes of respiratory illnesses in the returning traveler.
Gastrointestinal Illness
Gastrointestinal symptoms account for approximately one-third of returning travelers who seek medical attention. 25 Most diarrhea in travelers is self-limiting, with travelers diarrhea being the most common travel-related illness. 7 Diarrhea linked to travel in resource-poor areas is usually caused by bacterial, viral, or protozoal pathogens.
The most often encountered diarrheal pathogens are enterotoxigenic Escherichia coli and enteroaggregative E. coli , which are easily treated with commonly available antibiotics. 26 Physicians should be aware of emerging antibiotic resistance patterns across the globe. The CDC offers up-to-date travel information in the CDC Yellow Book . 3 Although patients are often concerned about parasites, they should be reassured that helminths and other parasitic infections are rare in the casual traveler. 3
The disease of concern in the setting of gastrointestinal symptoms is typhoid fever. Physicians should be aware that typhoid fever and paratyphoid fever are clinically indistinguishable, with cardinal symptoms of fever and abdominal pain. 3 Typhoid fever should be considered in ill returning travelers who do not have diarrhea, because typhoid infection may not present with diarrheal symptoms. The likelihood of typhoid fever also correlates with travel to endemic regions and should be considered an alternative diagnosis in patients not responding to antimalarial medications. A diagnosis of enteric fever can be confirmed with blood or stool cultures. Although less common, community-acquired Clostridioides difficile should be considered in the differential diagnosis in the setting of recent travel and potential antimicrobial use abroad. 27
Another important travel-related pathogen is hepatitis A due to its widespread distribution in the developing world and the small pathogen dose necessary to cause illness. Hepatitis A is a more serious infection in adults; however, many U.S. adults have been vaccinated because the hepatitis A vaccine is included in the recommended childhood immunization schedule. 28 eTable D lists the most common causes of gastrointestinal illnesses in the returning traveler.
Dermatologic Concerns
Dermatologic concerns are common among returning travelers and include noninfectious causes such as sun overexposure, contact with new or unfamiliar hygiene products, and insect bites. The most common infections in returning travelers with dermatologic concerns include cutaneous larva migrans, infected insect bites, and skin abscesses. Cutaneous larva migrans typically presents with an intensely pruritic serpiginous rash on the feet or gluteal region. 3 Questions about bites and bite avoidance measures should be asked of patients with symptomatic skin concerns; however, physicians should remember that many bites go unnoticed. 29
Formerly common illnesses in the United States are common abroad, with measles, varicella-zoster virus infection, and rubella occurring in child and adult travelers. 3 Measles is considered one of the most contagious infectious diseases. More than one-third of child travelers from the United States have not completed the recommended course of measles, mumps, and rubella vaccines at the time of travel due to immunization scheduling. One-half of all measles importations into the United States comes from these international travelers. 30 Measles should always be considered in the differential because of the low or incomplete vaccination rates in travelers and high levels of exposure in some areas abroad. eTable E lists the most common infectious causes of dermatologic concern in the returning traveler.
Data Sources: A PubMed search was completed using the key words prevention, diagnosis, treatment, travel related illness, surveillance, travel medicine, chemoprophylaxis, and returning traveler treatment. The search was limited to English-language studies published since 2000. Secondary references from the key articles identified by the search were used as well. Also searched were the Centers for Disease Control and Prevention and Cochrane databases. Search dates: September 2022 to November 2022, March 2023, and August 2023.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army, the U.S. Department of Defense, or the U.S. government.
The World Tourism Organization. International tourists to hit 1.8 billion by 2030. October 11, 2011. Accessed March 2023. https://www.unwto.org/archive/global/press-release/2011-10-11/international-tourists-hit-18-billion-2030
- Angelo KM, Kozarsky PE, Ryan ET, et al. What proportion of international travellers acquire a travel-related illness? A review of the literature. J Travel Med. 2017;24(5):10.1093/jtm/tax046.
Centers for Disease Control and Prevention. CDC Yellow Book: Health Information for International Travel . Oxford University Press; 2023. Accessed August 26, 2023. https://wwwnc.cdc.gov/travel/yellowbook/2024/table-of-contents
Wu HM. Evaluation of the sick returned traveler. Semin Diagn Pathol. 2019;36(3):197-202.
Scaggs Huang FA, Schlaudecker E. Fever in the returning traveler. Infect Dis Clin North Am. 2018;32(1):163-188.
Feder HM, Mansilla-Rivera K. Fever in returning travelers: a case-based approach. Am Fam Physician. 2013;88(8):524-530.
Giddings SL, Stevens AM, Leung DT. Traveler's diarrhea. Med Clin North Am. 2016;100(2):317-330.
Harvey K, Esposito DH, Han P, et al.; Centers for Disease Control and Prevention. Surveillance for travel-related disease–GeoSentinel Surveillance System, United States, 1997–2011. MMWR Surveill Summ. 2013;62:1-23.
Sridhar S, Turbett SE, Harris JB, et al. Antimicrobial-resistant bacteria in international travelers. Curr Opin Infect Dis. 2021;34(5):423-431.
Matteelli A, Carvalho AC, Bigoni S. Visiting relatives and friends (VFR), pregnant, and other vulnerable travelers. Infect Dis Clin North Am. 2012;26(3):625-635.
Ladhani S, Aibara RJ, Riordan FA, et al. Imported malaria in children: a review of clinical studies. Lancet Infect Dis. 2007;7(5):349-357.
Sanford C, McConnell A, Osborn J. The pretravel consultation. Am Fam Physician. 2016;94(8):620-627.
Shahbodaghi SD, Rathjen NA. Malaria. Am Fam Physician. 2022;106(3):270-278.
Freedman DO, Chen LH, Kozarsky PE. Medical considerations before international travel. N Engl J Med. 2016;375(3):247-260.
- Marti F, Steffen R, Mutsch M. Influenza vaccine: a travelers' vaccine? Expert Rev Vaccines. 2008;7(5):679-687.
Vivancos R, Abubakar I, Hunter PR. Foreign travel, casual sex, and sexually transmitted infections: systematic review and meta-analysis. Int J Infect Dis. 2010;14(10):e842-e851.
Paquet D, Jung L, Trawinski H, et al. Fever in the returning traveler. Dtsch Arztebl Int. 2022;119(22):400-407.
Cantey PT, Montgomery SP, Straily A. Neglected parasitic infections: what family physicians need to know—a CDC update. Am Fam Physician. 2021;104(3):277-287.
Rathjen NA, Shahbodaghi SD. Bioterrorism. Am Fam Physician. 2021;104(4):376-385.
Jensenius M, Davis X, von Sonnenburg F, et al.; Geo-Sentinel Surveillance Network. Multicenter GeoSentinel analysis of rickettsial diseases in international travelers, 1996–2008. Emerg Infect Dis. 2009;15(11):1791-1798.
Tolle MA. Evaluating a sick child after travel to developing countries. J Am Board Fam Med. 2010;23(6):704-713.
Centers for Disease Control and Prevention. About malaria. February 2, 2022. Accessed August 21, 2022. https://www.cdc.gov/malaria/about/index.html
Wilder-Smith A, Schwartz E. Dengue in travelers. N Engl J Med. 2005;353(9):924-932.
Summer A, Stauffer WM. Evaluation of the sick child following travel to the tropics. Pediatr Ann. 2008;37(12):821-826.
Swaminathan A, Torresi J, Schlagenhauf P, et al.; GeoSentinel Network. A global study of pathogens and host risk factors associated with infectious gastrointestinal disease in returned international travellers. J Infect. 2009;59(1):19-27.
Shah N, DuPont HL, Ramsey DJ. Global etiology of travelers' diarrhea: systematic review from 1973 to the present. Am J Trop Med Hyg. 2009;80(4):609-614.
Michal Stevens A, Esposito DH, Stoney RJ, et al.; GeoSentinel Surveillance Network. Clostridium difficile infection in returning travellers. J Travel Med. 2017;24(3):1-6.
Mayer CA, Neilson AA. Hepatitis A - prevention in travellers. Aust Fam Physician. 2010;39(12):924-928.
Herness J, Snyder MJ, Newman RS. Arthropod bites and stings. Am Fam Physician. 2022;106(2):137-147.
Bangs AC, Gastañaduy P, Neilan AM, et al. The clinical and economic impact of measles-mumps-rubella vaccinations to prevent measles importations from U.S. pediatric travelers returning from abroad. J Pediatric Infect Dis Soc. 2022;11(6):257-266.
Continue Reading

More in AFP
More in pubmed.
Copyright © 2023 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
Etiology of travel-related fever
Affiliation.
- 1 Harvard Medical School, Boston, Massachusetts, USA. [email protected]
- PMID: 17762776
- DOI: 10.1097/QCO.0b013e3282a95e27
Purpose of review: Many potentially life-threatening infections cause fever. Several recent large studies help to define causes of fever in returned travelers.
Recent findings: The destination of travel determines the relative likelihood of the different major causes of fever. Systemic febrile illness occurs disproportionately among ill travelers returning from sub-Saharan Africa. Malaria remains the most important overall cause of systemic febrile illness in travelers to tropical regions; dengue fever is now the most prominent cause of fever in travelers to certain regions, most notably Asia. Chikungunya fever has emerged as a major cause of fever in travelers to Indian Ocean islands off Africa and to India itself. Causes of fever vary by the time of presentation after travel. Vivax malaria is an important cause of fever with onset more than a month after return; recently studies have shown that parasites causing relapse are genetically distinct from those causing primary infection. At expert referral centers up to 25% of febrile patients have no specific cause of fever determined.
Summary: Knowledge of predominant causes of febrile infections by geographic region, traveler characteristics, and time of presentation can assist the clinician in guiding posttravel diagnosis and empiric therapy of ill returned travelers and is also valuable in pretravel preparation.
Publication types
- Alphavirus Infections / epidemiology*
- Chikungunya virus*
- Fever / etiology*
- Infections / epidemiology*
- Malaria / epidemiology*
We've detected unusual activity from your computer network
To continue, please click the box below to let us know you're not a robot.
Why did this happen?
Please make sure your browser supports JavaScript and cookies and that you are not blocking them from loading. For more information you can review our Terms of Service and Cookie Policy .
For inquiries related to this message please contact our support team and provide the reference ID below.

DOH reports another travel-related dengue case on Oʻahu

The Hawaiʻi Department of Health confirmed an additional dengue virus case on Thursday, bringing the statewide total to 11 this year.
One case was reported on Kauaʻi, three on Maui and seven on Oʻahu. All cases were travel-related.

Dengue is a viral illness that's spread by mosquitoes. Within a week of being bitten, symptoms may include high fever, headache, body aches, nausea and rash. However, some people may not have symptoms.
Dengue is not endemic to Hawaiʻi and cases are often seen in travelers.
Dengue outbreaks were have been reported in Central and South America, Asia, the Middle East, Africa and some Pacific Islands, including U.S territories.

Breaking News Alerts
Press "allow" to activate.
- Food & Dining
- Arts & Entertainment
- Real Estate
- Hawai‘i Journalism Initiative
- Crime Statistics
- Local Sports
- Weather Forecast
- Surf Report
- Maui Arts & Entertainment
- Food and Dining
- On the Menu
- Visitors’ Guide
- Maui Discussion
- Reader Survey
- Upcoming Maui Events
- Map of Events
- Post an Event
- Recent Job Listings
- IMUA Discovery Garden
- Medical Minute
- Latest Maui Videos
- About Maui Now
- Contact Information
- Get the App
- Advertising
- Meet the Team
Privacy Policy | About Our Ads

- Entertainment
DOH reports travel-related dengue case on Oʻahu, brining state total to 11 in 2024
The Hawai‘i Department of Health has identified a new case of travel-related dengue virus case on Oʻahu, bringing the number of total cases to 11 identified in the state in 2024 (one on Kauaʻi, three on Maui, seven on Oʻahu). The affected travelers were exposed in various countries where dengue is common.
DOH teams were deployed for inspection and mosquito control in the affected area. The community is asked to help reduce the risk for any local transmission by exercising best practices described below.
Dengue virus is spread from infected person to mosquito to person. While Hawai‘i is home to the type of mosquitoes that can carry dengue, the disease is not endemic (established) here in the state and cases are currently only seen in travelers. Multiple regions around the world are currently experiencing higher-than-normal dengue activity.
Dengue outbreaks do occur in many parts of the world including Central and South America, Asia (including the Republic of the Philippines), the Middle East and Africa, as well as some Pacific Islands including the US territories of American Samoa, the Federated States of Micronesia, the Republic of Marshall Islands and the Republic of Palau, in addition to many popular tourist destinations in the Caribbean (including Puerto Rico).
Anyone who plans to travel or has traveled to an area with dengue is at risk for infection. Currently, the Centers for Disease Control and Prevention (CDC) advises travelers to practice usual precautions when traveling to areas of dengue risk. T
Symptoms of dengue typically may be mild or severe and include fever, nausea, vomiting, rash and body aches. Symptoms typically last two to seven days and although severe and even life-threatening illness can occur, most people recover after about a week.
In areas of suspected or confirmed dengue, Hawai‘i DOH personnel (Vector Control Branch) conduct inspections and mosquito-reducing activities. Reducing mosquito populations reduces the chances of dengue being transmitted to other people. In areas without reported dengue cases, eliminating mosquito breeding sites in and around your home is a good practice. Mosquitoes only need small amounts of standing water to breed. Common breeding sites at home include buckets, water-catching plants (such as bromeliads), small containers, planters, rain barrels, or even cups left outside. Simply pouring out containers of standing water eliminates the potential for mosquito breeding.
For more information, visit the Disease Outbreak Control Division (DOCD) website and Vector Control Branch (VCB) website .
Sponsored Content

Subscribe to our Newsletter
- Send Me Daily Updates
- Send Me Weekly Updates

- Maui Business
- Maui Sports
- Maui Activities
- Maui Events Calendar
- Official Visitors’ Guide
- About Our Ads
- Terms of Service

Facebook YouTube Twitter Instagram
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Environ Res Public Health

Travel-Related Typhoid Fever: Narrative Review of the Scientific Literature
Narcisa muresu.
1 Department of Biomedical Sciences, University of Sassari, 07100 Sassari, Italy; moc.kooltuo@userumasicran
Giovanni Sotgiu
2 Department of Medical, Surgical and Experimental Sciences, University of Sassari, 07100 Sassari, Italy; ti.ssinu@ussocaerdna (A.C.); ti.ssinu@irottedam (M.D.); ti.ssinu@araza (A.A.); ti.ssinu@iredasl (L.S.); ti.ssinu@anaip (A.P.)
Bianca Maria Are
3 Hygiene and Preventive Medicine Unit, AOU Sassari, 07100 Sassari, Italy; ti.ssinu@mbera
Andrea Cossu
Clementina cocuzza.
4 Department of Medicine and Surgery, University of Milano-Bicocca, Via Cadore 48, 20900 Monza, Italy; [email protected] (C.C.); [email protected] (M.M.)
Marianna Martinelli
Sergio babudieri.
5 Infectious Diseases Department, AOU Sassari, University of Sassari, 07100 Sassari, Italy; ti.ssinu@redubab (S.B.); [email protected] (R.A.)
Riccardo Are
Marco dettori, antonio azara, laura saderi, andrea piana.
Enteric fever is a foodborne infectious disease caused by Salmonella enterica serotypes Typhi and Paratyphi A, B and C. The high incidence in low income countries can increase the risk of disease in travelers coming from high income countries. Pre-travel health advice on hygiene and sanitation practices and vaccines can significantly reduce the risk of acquiring infections. Although the majority of the cases are self-limiting, life-threatening complications can occur. Delayed diagnosis and cases of infections caused by multi-drug resistant strains can complicate the clinical management and affect the prognosis. More international efforts are needed to reduce the burden of disease in low income countries, indirectly reducing the risk of travelers in endemic settings. Surveillance activities can help monitor the epidemiology of cases caused by drug-susceptible and resistant strains.
1. Introduction
In the context of a globalization process, travels can pose a threat to the health of millions of persons worldwide. Outbreaks and epidemic episodes of transmissible diseases (e.g., Ebola, Zika, Middle East respiratory syndrome, etc.), potentially associated with travel of population groups, have raised the attention of supranational and national governments based on their mortality, morbidity, and impact on the sustainability of country and regional healthcare systems. Migration waves and business or holiday travels might be the epidemiological driver of several infectious diseases in low incidence geographical areas: involvement of numerous contagious and susceptible individuals, as well as rapid transfer of patients through modern transportation means, might create the epidemiological conditions for an unforeseen outbreak. Infectious diseases, rare in some geographic areas, can occur and rapidly spread in the context of unprepared national healthcare systems.
In addition, the number of international travelers is increasing globally and will be presumptively 1800 million by 2030 [ 1 ]. In total, 1326 million and 1401 million international travelers were recorded in 2017 and 2018, respectively. Between 2007 and 2011, infectious diseases were diagnosed in 42,173 travelers coming from Asia (32.6%), Sub-Saharan Africa (26.7%), and Latin America and the Caribbean (19.2%). Some of the infectious diseases were systemic (e.g., malaria, dengue, and enteric fever) and about one-third were caused by gastro-intestinal pathogens (e.g., Campylobacter spp., Salmonella spp., and Shigella spp.) [ 2 , 3 ]. Whereas a decreasing trend of incident malaria has been recorded from 2000 to 2015, incidence of enteric and dengue fever has not changed overtime [ 4 ].
Enteric fever, which includes Typhoid and Paratyphoid fever, is an infectious disease caused by Salmonella enterica serotypes Typhi and Paratyphi A, B and C. Their foodborne transmission, frequently associated with poor hygiene conditions and inadequate sanitation, favors outbreaks in low income countries [ 5 ]. Based on the most recent global estimates, ≥21 million incident cases and 222,000 typhoid-related deaths occur annually [ 6 ]. Improved sanitation and living conditions, as well as treatment of drinking water, have significantly contributed to decrease the incidence of enteric fever in high income countries (e.g., those located in Western Europe and North America). The Indian subcontinent and Southeast Asia show the highest annual incidence of typhoid fever (>100 cases per 100,000 cases annually), followed by Southern Africa (10–100 per 100,000 cases annually) [ 7 , 8 ]. In a recent meta-analysis conducted by Marchello and Colleagues [ 9 ], Africa and Asia were identified as high-endemic countries for typhoid fever, although a decreased trend in incidence was documented after 2000. Moreover, in low-resource areas, such as Tanzania, Myanmar, and Republic Democratic of Congo (DRC), S. typhi represents the leading cause of bloodstream infections in young children. In particular,>70% of cases occurred in children <10 years old and ~30% in <5 years old in DRC during 2015–2017. However, in high income countries, typhoid fever is one of the most frequently diagnosed vaccine-preventable diseases in returned international travelers and migrants coming from high incidence countries [ 10 , 11 ].
It has been estimated that the incidence rate of typhoid fever in travelers to high-endemic countries is 3–30 cases per 100,000 travelers [ 12 ].
A retrospective study carried out in the Netherlands from 1997 to 2014 found that the majority (59.6%) of patients with imported typhoid fever traveled in Asia (e.g., Indonesia (19.8%) and India (19.6%)), and Morocco (13.3%). A declining annual attack rate (i.e., annual incidence of imported cases to number of travelers in a geographical area) for all geographical destinations, with the only exception of India, has been described [ 13 ].
The more frequently affected age group was 25–29 years according to the findings of a survey performed in Australia, which confirmed East and South Asia as the highest risk geographical areas for individuals visiting their country of birth [ 14 ].Similar findings were confirmed by a Greek study which highlighted the risk of traveling in the Indian subcontinent during 2004–2011 (83.3% of the cases of travel-associated enteric fever), especially in VFR (Visiting Friends and Relatives)-travelers, whose disease is associated with longer stay, exposure to contaminated water and food, and difficult access to pre-travel medical services due to language and cultural barriers, as well as to lower rates of vaccination against travel-related preventable infections, including typhoid vaccine [ 15 , 16 ]. Similarly, a retrospective study conducted in Qatar, between 2005 and 2012, reported 356 cases of typhoid fever, of whom 96.9% had traveled abroad, mainly in the Indian subcontinent [ 17 ]. Over 70% of typhoid fever cases in Europe are acquired abroad and frequently caused by strains with marked antibiotic resistance profile [ 18 , 19 ].
In Italy, where typhoid fever was endemic in the first half of the last century, the mean annual notification rate was 127.6 cases during2007–2016. Although all cases were successfully treated, an unequal distribution of incident cases in the population group aged 25–44 years was found, likely linked to their travel habits [ 20 ].
When traveling from high- to low- and middle-income countries, the risk of infectious diseases is higher in VFR-travelers, followed by travelers for other reasons. Migrants from low income countries represent a vulnerable population group at highest risk of respiratory, vector- and food-borne diseases owing to the higher circulation of microorganisms in their country of origin. Moreover, the higher risk could depend on long periods of stay in the country of origin, often in remote rural areas where the healthcare infrastructures are poor, and on close contact with the local population, as well as on consumption of local food and water [ 21 ]. Frequent travels from/to high incidence countries increase the probability of acquiring infections, such as those caused by Mycobacterium tuberculosis , HIV, Plasmodium spp., and Salmonella spp. Ten years of surveillance in the UK demonstrated lower rates of enteric fever in UK-born vs. migrant populations. Migrants from South Asian countries are at highest risk of enteric fever (80% of the migrant cases) [ 22 ].
Another group at highest risk includes persons involved in humanitarian staffing (e.g., missionaries, medical, and humanitarian workers): their length of stay is long and their travel destinations are low income countries where the incidence of the above-mentioned infectious diseases is high. Nevertheless, the Global TravEpi Network (GTEN) data in US showed an appropriate pre-travel care and vaccination, over 90% of coverage, for hepatitis A, typhoid, and yellow fever [ 23 ].
Although epidemiological data revealed that the occurrence of typhoid fever cases in high-income countries is a rare event and the awareness, as well as the knowledge, of the disease is poor, up-to-date estimates of typhoid fever incidence could be useful in supporting prevention and vaccination national strategies. Moreover, it should be helpful to identify groups at high risk of infection to plan adequate preventive strategies. In addition, based on the poor specificity of typhoid fever symptoms, the potential diagnostic delay could increase the risk of a rapid spread in low-incidence areas.
A non-systematic, narrative review to retrieve the scientific evidence on imported enteric (i.e., typhoid and paratyphoid) fever diagnosed in high income countries was carried out to describe relevant clinical and public health features. The search engine PubMed was used to select peer-reviewed articles, published from 1January 2013 to 30 October 2019. References of the selected manuscripts were carefully assessed to detect important articles not included in the primary search. No detailed selection criteria were adopted to choose the articles. The following keywords were used to find articles on imported enteric fever-related diagnosis, therapy, epidemiology, and prevention: “typhoid fever”, “enteric fever”, and “travel”. In total, 207 records, published between January 2013 and October 2019, were found. Based on titles, abstracts, and full-texts, 71 (34.3%) studies were deemed suitable. Twenty-three (32.4%) were focused on epidemiological characteristics of enteric fever, fourteen (19.7%) on vaccines, fourteen (19.7%) on antimicrobial resistance of Salmonella spp. serotypes, ten (14.1%) on population groups at higher risk of acquiring typhoid fever, and ten (14.1%) on diagnosis and treatment.
2.1. Population at Higher Risk of Acquiring Typhoid Fever
The risk of typhoid fever and non-typhoidal Salmonella invasive infections is highest in infants, young children, and young adults with underlying comorbidities, including severe anemia, malaria, malnutrition, and HIV infection [ 24 ]. Moreover, recent reports from the international travelers agency showed that immunocompromised travelers, who usually follow the same itineraries of immunocompetent persons, visit countries at high risk of infections but the risk of developing travel-related diseases is five times higher if compared with that of immunocompetent persons [ 25 ].
However, data on groups at risk of acquiring typhoid infections are controversial and scant. Gordon showed that the immunological status cannot be associated with an increased risk or poor outcome. However, invasive diseases caused by non-typhoidal salmonellae are more frequently diagnosed in immunocompromised persons (e.g., persons with HIV/AIDS) [ 26 ]. Likewise, a study conducted in Africa did not find differences in HIV-positive patients and controls in the clinical presentation and outcomes of typhoid fever cases [ 27 ]. In contrast, Gotuzzo and Colleagues [ 28 ] found a rate of typhoid fever 25 times higher in HIV-positive patients than in the general population.
With the remarkable increased number of travelers from high-income countries during the last two decades, it was estimated that 1.9 million children traveled overseas every year from the United States; similarly, a significant increase of travelers (1.7 fold) was shown in Greece from 2004 to 2008 [ 29 ]. A high proportion of enteric fever cases was described in children aged 0–14 years (>26% in 2018) [ 30 ], mainly attributed to tourism and VFR-travels. Zhou and Colleagues highlighted an increased rate of childhood enteric fever in a large tertiary care center in Canada during 1985–2013, with several cases caused by Salmonella paratyphi A and B and by bacterial strains resistant to first-line antibiotics [ 31 ]. In Australia, 87% of the childhood cases were acquired mainly in Southeast Asia, with an annual increasing incidence from the period 2001–2005 (13 cases per year) to the period 2011–2015 (38 cases per year) [ 32 ]. Similar data were described in France, where children aged <18 years accounted for one-third of enteric fever patients, with 61% of the infections acquired in Africa [ 33 ].
Pre-travel counseling focused on hygiene and preventive measures could help reduce the risk of infection in individuals younger than two years, who cannot be immunized with the currently available vaccines [ 29 ].
2.2. Vaccination Status in Travelers
Infections can be averted with vaccines and hygiene-related recommendations. However, adherence to pre-travel advice, including the vaccination, is poor.
Since 2008, the World Health Organization has advocated the control of typhoid fever based on vaccine-related strategies. Bill and Melinda Gates Foundation launched in 2017 a partnership called “Typhoid Fever Vaccine Acceleration Consortium (TyVAC)”, mainly focused on children living in high endemic areas, to increase the prescription of the typhoid conjugate vaccine in Africa and Asia [ 34 ].
Currently, three typhoid vaccines are available:
- Live attenuated oral vaccine Ty21a
- Purified Vi capsular polysaccharide injectable vaccine
- Purified Vi polysaccharide conjugated parenteral vaccine
The effectiveness of the current vaccination strategies in travelers depends on several variables, such as previous immunizations, type, and length of travel. Poor awareness on the high risk of foodborne diseases in low- and low-middle-income countries can increase the pool of individuals with a vaccine hesitancy.
Surveys conducted in EU/EEA countries showed that hepatitis A is the first vaccine administered to Swiss (53%) and Italian (63%), travelers, followed by tetanus–diphtheria in Swiss (45.6%) and typhoid-fever vaccine in Italian (44.6%) travelers. Moreover, travel destinations can increase the request of pre-travel care (e.g., India and Thailand chosen for pleasure or business) [ 35 , 36 ]. Furthermore, low vaccine uptake and inappropriate precautions adopted by VFR-travelers was associated with a highest incidence of foodborne diseases [ 36 , 37 ].
A Greek survey focused on the administration of typhoid fever vaccines showed that a high proportion (44.2%) of travelers to India accepted the prescription of a vaccine, in comparison with a lower percentage of individuals traveling to Africa (~31%) [ 37 ].
Ty21a live attenuated vaccine, developed from attenuated Ty2 strains of S. typhi , does not confer protection after a single dose; three doses administered in alternate days in persons living in endemic countries and four doses in travelers are usually recommended to elicit adapted mucosal immunity (IgA antibodies), whose duration lasts ~7 years in 60–70% of the vaccinated cases [ 38 , 39 ]. Half of typhoid fever cases could be prevented until three years after vaccination [ 40 ].
Purified Vi capsular polysaccharide vaccine, administered in one single dose, is associated with a high immunogenicity (80–95%) in adults and children older than two years; nevertheless, Anwar and Colleagues reported a preventive effect between one third and one half of the cases in the first two years after the vaccination and no clear benefits after three years [ 41 ]. Moreover, an acceptable immunogenicity and safety profile was shown when co-administered with yellow-fever and quadrivalent meningococcal vaccines [ 42 ], as well as in children and HIV-positive individuals [ 38 ]. A second dose is recommended after three years [ 39 ].
Purified Vi polysaccharide vaccine can be conjugated to toxoids (Vi-diphtheria, Vi-Tetanus, and Vi-recombinant diphtheria CRM 197 ) to increase IgG levels [ 38 ]. Two-year post-vaccination effectiveness was87% [ 36 , 43 ]. A single randomized trial in Indian children aged between six months and 12 years did not clarify the efficacy of two doses of Vi-TT vaccine one year after administration [ 40 ].A real-life survey in Germany described an adverse event rate of 28.6% (fatigue, pain, headache, pyrexia, myalgia, and swelling), increased after concomitant immunization with other vaccines(i.e., rabies, typhoid, and yellow fever vaccines) [ 44 ].A US military study showed more adverse events in individuals exposed to the polysaccharide Vi vaccine in comparison with those exposed to the oral vaccine, although rash and diarrhea were more incident in the latter group [ 45 ].Fever and pain at the injection site were incident using parenteral vaccines [ 40 ].
Several studies suggested a cross-protection against S. paratyphi A, B and C with the administration of the oral vaccine Ty21a (common O- and Vi-antigens) [ 38 ], even if a study which recruited US military personnel described a weak immunity against S. paratyphi A [ 46 ].
Primary prevention based on the available vaccines cannot be implemented in youngest children: the purified Vi capsular polysaccharide vaccine is not recommended in children aged <2 years for its poor immunogenicity; the live attenuated oral vaccine Ty21a is not well tolerated in children aged under five years [ 47 ].A recent review on the efficacy of the currently available vaccines confirmed that Ty21a and Vi polysaccharide vaccines can reduce the incidence of infections in adults and children aged >2 years [ 40 ].
2.3. Clinical Diagnosis and Treatment of Typhoid Fever
The clinical management of typhoid fever in travelers has significantly changed during the last two decades following the widespread distribution of MDR bacteria and the increased number of international travels in endemic countries, including high-risk individuals (e.g., children, immunocompromised patients, pregnant women, and elderly people) [ 48 ]. Although illness in travelers is usually not severe and self-limiting, urgent therapy can be needed to avoid life-threatening complications; then, a rapid diagnosis and therapy in returning travelers is key to avoid fatal consequences [ 49 ]. The information on the type of travel, including the collection of details such as accommodation and activities, as well as pre-travel immunization, could help in the differential diagnosis (e.g., malaria and dengue). The most prevalent symptoms in the case of typhoid infections are fever, diarrhea, vomiting, abdominal pain, and headache [ 31 , 50 ]. However, the specificity is poor and they can be attributed to other viral or bacterial agents, in both children and adults. Moreover, higher level of C-reactive protein can be also found in case of dengue and malaria. Typhoid fever is commonly characterized by gastrointestinal disorders [ 51 , 52 , 53 ] and, moreover, bradycardia (88%), and eosinopenia (63%) [ 54 ]. Empirical therapy is needed in the case of severe symptoms and when a rapid diagnosis cannot be performed.
As recommended by the international guidelines [ 24 ], a definitive diagnosis of typhoid fever requires cultural or molecular methods, with specimens collected ideally before the administration of an antimicrobial therapy. Blood is the preferred specimen. Although serologic tests are frequently requested, several studies highlighted their poor specificity and sensitivity. Antimicrobial susceptibility testing is strongly recommended for clinical (prescription of a tailored antibiotic therapy) and public health (surveillance) purposes.
Epidemiological investigations are key for the identification of the source case and of the contagious patients. Serotyping carried out by slide agglutination method and the characterization of the genomic profile using Pulsed Field Gel Electrophoresis (PFGE) and Multi Locus Sequence Typing (MLST) are recommended to confirm epidemiological links during a suspected outbreak.
In the era of multidrug resistant strains, the adoption of antimicrobial susceptibility testing is necessary to guide the choice of the most appropriate therapy, improving the clinical management, preventing relapse and the chronic carrier status. Europeans Committee on Antimicrobial Susceptibility Testing (EUCAST) and Clinical and Laboratory Standards Institute (CLSI) recommend the use of the following antibiotics to be tested in vitro for infections caused by S. typhi and S. paratyphi : ampicillin, chloramphenicol, cotrimoxazole, ciprofloxacin, ceftriaxone, and azithromycin ( Table 1 ).
Clinical breakpoints recommended by EUCAST and CLSI. (NA, Not Applicable).
Patients are usually treated with oral antibiotics, antipyretic, and supportive therapy. Parenteral antibiotics are prescribed in complicated cases or when gastro-intestinal symptoms are persistent. Ciprofloxacin (15 mg/kg) for 5–7 days is prescribed in the case of moderate symptoms caused by fully susceptible isolates [ 55 ]. With the emergence of multidrug resistant strains, the first line treatment was represented by third generation cephalosporins, such as cefixime (20 mg/kg, max 200 mg) for 10–14 days. Alternative drugs are chloramphenicol (50–75 mg/kg/day) for 14–21 days, trimethoprim-sulfamethoxazole (8 mg/kg/day) for 14 days, or amoxicillin (75–100 mg/kg/day) for 14 days [ 56 ]. Cases of severe enteric fever, characterized by delirium, stupor, coma, or obtundation, can be successfully treated with corticosteroids (e.g., intravenous dexamethasone at an initial dose of 3 mg/kg, followed by 1 mg/kg every six hours for two days) [ 57 ].
2.4. Antimicrobial Resistance and Typhoid Fever
In the case of typhoid fever, the World Health Organization recommends notification to national authorities and drug susceptibility testing.
Until multidrug resistant strains emerged and spread in the second half of the 1980s, chloramphenicol, ampicillin, and co-trimoxazole were considered the first-line therapy of typhoid fever globally [ 58 ]. The indiscriminate use of antibiotics, particularly in low-income areas, has favored the selection and spread of antimicrobial resistant strains [ 59 ].
The first outbreak caused by chloramphenicol-resistant S. typhi occurred in Mexico in 1972, followed by notifications in other endemic areas (i.e., South Asia andAfrica) [ 60 ]. Moreover, ~66% of isolates in European countries [ 17 ], shows decreased susceptibility to ciprofloxacin, although it was considered in the past as first therapeutic choice [ 61 ] by the World Health Organization guidelines. In U.S., during 2016–2018, 5/29 cases of typhoid fever in patients who recently traveled to Pakistan were caused by extensively drug resistant strains [ 18 ].
Data of the US antimicrobial monitoring system showed that >60% of the isolates had a reduced susceptibility to ciprofloxacin and nalidixic acid, as well as alarming increasing trends of resistance of S. typhi and paratyphi A [ 62 ] ( Table 2 ).
Antimicrobial resistance pattern for Salmonella ser. Typhi (No. of isolates = 336), Paratyphi A (No. of isolates = 88), 2015 (CDC-National Antimicrobial Resistance Monitoring System-2015).
Britto and Colleagues systematically reviewed historical trends of drug resistance to first-line antimicrobial therapy in typhoid fever (1973–2018, for 13,833 isolates) ( Table 3 ).
Proportion (%) of drug-resistant isolates of Salmonella typhi .
The majority were isolated in South Asia (63.2%), Africa (15%), and Southeast Asia (12.8%). Moreover, during 2006–2015, a significant increasing trend of drug resistance was described for the nalidixic acid and fluoroquinolones [ 63 ].
The increasing rate of S. typhi isolates resistant to quinolones, ampicillin, and cotrimoxazole prompted a policy change in terms of antibiotic prescription, mainly focused on third-generation cephalosporines [ 31 , 32 ]. Strains of multi-drug resistant (MDR) Salmonella typhi with the haplotype H58 were found worldwide, and accounted for multiple outbreaks in endemic countries and single cases in international travelers. Moreover, Klemm and Colleagues [ 64 ] detected several resistance genes (extended-spectrum β -lactamase genes, and ESBLs) in extensively-drug resistant strains, previously detected in other enteric bacteria [ 65 ]. Resistance is mainly associated with cumulative mutations in quinolone resistance-determining regions (QRDR) encoding DNA gyrase ( gyrA and gyrB genes) and topoisomerase ( parC and parE genes) [ 61 , 66 ]. A recent study, which analyzed >500 isolates of S. typhi , found that the reduced susceptibility to fluoroquinolones is frequently (85%) associated with point mutations in the QRDR; however, the prevalence can vary depending on the geographical area:95% in South Asia, 43% in EastAfrica, and 27% in WestAfrica. Strains resistant to ciprofloxacin were mainly collected from patients diagnosed in India, accounting for 23% of the total Indian cases [ 67 ].
Qnr -family genes, which are responsible of plasmid-mediated quinolone resistance, confer resistance to nalidixic acid and are recommended as a surrogate marker of decreased susceptibility to ciprofloxacin [ 66 ].
Recent reports from high-income countries described trends of decreased susceptibility for several antimicrobial drugs [ 61 , 66 , 68 ]: ciprofloxacin-resistant strains were 63%, 55.6%, and ~43% in Italy, Switzerland, and Spain, respectively. Antimicrobial resistance is frequently reported in USA where 69% of strains isolated between 2008 and 2012 were resistant to nalidixic acid or showed reduced susceptibility to ciprofloxacin, 12% were MDR, and 10% showed extensive drug resistance [ 69 ]. Furthermore, the poor antimicrobial susceptibility was found in both S. parathypi and S. typhi isolates; drug resistance to quinolones was detected in 66.7% and 20% isolates of S. paratyphi and S. typhi , respectively [ 68 ].
Third generation cephalosporins (e.g., ceftriaxone) are successfully prescribed for infections caused by ciprofloxacin-resistant strains; alternatively, meropenem and aztreonam can be administered, even if the clinical breakpoint for aztreonam is not currently available [ 70 ]. Furthermore, 354 isolates of S. typhi collected in the Netherlands showed resistance to ciprofloxacin and azithromycin, reducing the availability of effective therapeutic options, mainly in travelers with acquired infections in endemic settings [ 71 ].
On this basis, World Health Organization recommends the management of sporadic cases of typhoid fever diagnosed in high-income countries be performed in reference centers, where a comprehensive assessment, including the complete drug susceptibility testing, could be carried out [ 24 ].

3. Conclusions
The present narrative review highlights that typhoid fever is an important clinical and public health issue in returning travelers. More international efforts and cooperation activities should be planned to reduce the burden of foodborne diseases, including typhoid fever, in low- and middle-income countries, addressing the key role played by the global surveillance systems. In particular, reliable and real-time estimates of typhoid fever can help better assess the efficacy and cost-effectiveness of ad hoc activities realized in both low and high endemic countries. The knowledge of the healthcare workers on travel related-diseases should be improved, focusing on the importance of a rapid diagnosis, including a rapid drug susceptibility testing, for the identification of difficult-to-treat cases (e.g., patients with typhoid fever caused by multi-drug resistant strains).
Primary prevention strategies and pre-travel health advices could decrease the risk of acquiring infections: improved awareness in community members could support and improve the adherence, mainly in highly susceptible groups (e.g., children and immunocompromised patients).
Nevertheless, further epidemiological studies should be focused on the effectiveness of currently available vaccines in population groups poorly evaluated in studies and trials carried out in the past. International surveillance networks involving endemic countries are needed to rapidly detect the emergence and spread of drug-resistant bacteria and decrease the incidence of imported cases in high-income countries. The financial and economic impact of endemic diseases can be easily reduced improving hygiene conditions in constrained-resource countries; however, only the improvement of political and economic issues can be achieved with comprehensive interventions supported by the international community, which directly and indirectly can affect the epidemiology of life-threatening diseases.
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
NEWS... BUT NOT AS YOU KNOW IT
Holiday spot that welcomed 33,000,000 tourists last year tops ‘heat death list’

Share this with

Europe’s top tourist destinations have been rated ‘extreme’ for the risk of heat death.
Greece is at the top of the list of the deadliest places amid temperatures reaching well above the 40°C mark.
This is all the while wildfires swept closer to the capital Athens , lighting the sky above the Acropolis orange in apocalyptic scenes .
Mediterranean destinations such as Italy and Spain are also among nations with most fatalities due to the heat.

Greece came out worst for heat-related deaths with 393 per one million residents, followed by Bulgaria (229), Italy (209) and Spain (175).
Travel warnings to UK holidaymakers have been issued because of the heatwave sweeping Europe, where temperatures have pushed above 40°C.
An EU-funded map setting out which areas are at high risk for heat death shows the entire of Italy graded purple for ‘extreme’ today, while the north of Greece is also at the highest level.
All of Bulgaria is at the highest level, while popular cities in Spain including Barcelona and Malaga are also on high alert.
Forecaster.health, a freely accessible health warning system for 580 regions in 31 European countries, shows how some European holiday hotspots may be best avoided, or should be visited with great care and plenty of water.

Barcelona-based IS Global, which created the map, said: ‘Ambient temperatures are associated with more than 5 million premature deaths a year worldwide, more than 300,000 of them in Western Europe.
‘In a context of rapid warming where temperatures have successively broken previous records over the past two decades, it is essential to use epidemiological models to develop novel early warning systems that anticipate the effects of expected temperatures on health.’
Latest London news
- UK braced for 'hottest day of the year'
- Rents are still skyrocketing in London — would a cap make a difference?
- Firefighters save ducklings who got stuck down a manhole
To get the latest news from the capital visit Metro.co.uk's London news hub .
In high temperatures, governments prepare for a spike in heat-related deaths, as bodies must work harder to stay at a safe temperature, making heart attacks and strokes more likely as well as worsening asthma and lung conditions.
These effects begin to kick in above 25 ° C or 26 ° C, with around 2,000 deaths in hot weather each year on average in England.
Heat alerts were in place here during the mini heatwave we had in July, with elderly people at risk as well as those with health conditions.
That was only with temperatures around 30°C at maximum, so when the mercury starts to push 40°C the health risk becomes much more severe.
Holiday hotspots with 'extreme' heat risk today
- All of Italy, including Sicily and Sardinia
- All of Bulgaria
- Northern Greece
- Some parts of western Greece
- Greek islands including Rhodes and Kos
- Malaga, Spain
- Barcelona, Spain
To use the map, enter the date you want health predictions for up to two weeks in advance, as well as the population group you want information for.
The map will then be colour-coded by which areas are at risk from heat or cold deaths:low, moderate, high and extreme.
It is based on temperature records and forecasts and uses epidemiological models to quantify the risk of temperature-related mortality by sex and age group.
Erika Radford, from Asthma + Lung UK, said: ‘Hot summer weather can bring on asthma symptoms like chest tightness, coughing, breathlessness and wheezing for some people.
‘The exact causes are not fully understood, but it’s thought that the warmer air may cause the airways to narrow. Additionally, during hot weather, there tends to be an increase in pollen levels, which can trigger potentially life-threatening asthma attacks.
‘Hot and humid weather can also worsen symptoms in people with other lung conditions, like chronic obstructive pulmonary disease (COPD), especially if you become dehydrated.
To find out the data for a specific date, use the interactive map on the website here.
Get in touch with our news team by emailing us at [email protected] .
For more stories like this, check our news page .
MORE : Sloth fever map shows where deadly disease is spreading across Europe
MORE : New suction-mouthed species which lived in UK 1.7million years ago discovered
MORE : The devastating reason this man is travelling 50,000km to deliver 50 letters
Sign Up for News Updates
Get your need-to-know latest news, feel-good stories, analysis and more.
Privacy Policy

Get us in your feed
You are using an outdated browser. Upgrade your browser today or install Google Chrome Frame to better experience this site.
Guatemala Traveler View
Travel health notices, vaccines and medicines, non-vaccine-preventable diseases, stay healthy and safe.
- Packing List
After Your Trip
Be aware of current health issues in Guatemala. Learn how to protect yourself.
Level 1 Practice Usual Precautions
- Updated Global Dengue August 14, 2024 Dengue is a year-round risk in many parts of the world, with outbreaks commonly occurring every 2–5 years. Travelers to risk areas should prevent mosquito bites. Destination List: Afghanistan, and Austral Islands (Tubuai and Rurutu), and Bora-Bora), Brazil, Burkina Faso, Cape Verde, Colombia, Costa Rica, Cuba, Ecuador, including the Galápagos Islands, El Salvador, French Guiana (France), French Polynesia, including the island groups of Society Islands (Tahiti, Ghana, Guatemala, Guyana, Honduras, Iran, Laos, Mali, Marquesas Islands (Hiva Oa and Ua Huka), Mauritius, Mexico, Moorea, Panama, Samoa, Singapore, Sri Lanka, Sudan, Uruguay
⇧ Top
Check the vaccines and medicines list and visit your doctor at least a month before your trip to get vaccines or medicines you may need. If you or your doctor need help finding a location that provides certain vaccines or medicines, visit the Find a Clinic page.
Routine vaccines
Recommendations.
Make sure you are up-to-date on all routine vaccines before every trip. Some of these vaccines include
- Chickenpox (Varicella)
- Diphtheria-Tetanus-Pertussis
- Flu (influenza)
- Measles-Mumps-Rubella (MMR)
Immunization schedules
All eligible travelers should be up to date with their COVID-19 vaccines. Please see Your COVID-19 Vaccination for more information.
COVID-19 vaccine
Chikungunya
There has been evidence of chikungunya virus transmission in Guatemala within the last 5 years. Chikungunya vaccination may be considered for the following travelers:
- People aged 65 years or older, especially those with underlying medical conditions, who may spend at least 2 weeks (cumulative time) in indoor or outdoor areas where mosquitoes are present in Guatemala, OR
- People planning to stay in Guatemala for a cumulative period of 6 months or more
Chikungunya - CDC Yellow Book
Hepatitis A
Recommended for unvaccinated travelers one year old or older going to Guatemala.
Infants 6 to 11 months old should also be vaccinated against Hepatitis A. The dose does not count toward the routine 2-dose series.
Travelers allergic to a vaccine component should receive a single dose of immune globulin, which provides effective protection for up to 2 months depending on dosage given.
Unvaccinated travelers who are over 40 years old, immunocompromised, or have chronic medical conditions planning to depart to a risk area in less than 2 weeks should get the initial dose of vaccine and at the same appointment receive immune globulin.
Hepatitis A - CDC Yellow Book
Dosing info - Hep A
Hepatitis B
Recommended for unvaccinated travelers younger than 60 years old traveling to Guatemala. Unvaccinated travelers 60 years and older may get vaccinated before traveling to Guatemala.
Hepatitis B - CDC Yellow Book
Dosing info - Hep B
CDC recommends that travelers going to certain areas of Guatemala take prescription medicine to prevent malaria. Depending on the medicine you take, you will need to start taking this medicine multiple days before your trip, as well as during and after your trip. Talk to your doctor about which malaria medication you should take.
Find country-specific information about malaria.
Malaria - CDC Yellow Book
Considerations when choosing a drug for malaria prophylaxis (CDC Yellow Book)
Malaria information for Guatemala.
Cases of measles are on the rise worldwide. Travelers are at risk of measles if they have not been fully vaccinated at least two weeks prior to departure, or have not had measles in the past, and travel internationally to areas where measles is spreading.
All international travelers should be fully vaccinated against measles with the measles-mumps-rubella (MMR) vaccine, including an early dose for infants 6–11 months, according to CDC’s measles vaccination recommendations for international travel .
Measles (Rubeola) - CDC Yellow Book
Dogs infected with rabies are sometimes found in Guatemala.
Rabies is also present in bats.
If rabies exposures occur while in Guatemala, rabies vaccines may only be available in larger suburban/urban medical facilities.
Rabies pre-exposure vaccination considerations include whether travelers 1) will be performing occupational or recreational activities that increase risk for exposure to potentially rabid animals and 2) might have difficulty getting prompt access to safe post-exposure prophylaxis.
Please consult with a healthcare provider to determine whether you should receive pre-exposure vaccination before travel.
For more information, see country rabies status assessments .
Rabies - CDC Yellow Book
Recommended for most travelers, especially those staying with friends or relatives or visiting smaller cities or rural areas.
Typhoid - CDC Yellow Book
Dosing info - Typhoid
Yellow Fever
Required for travelers ≥1 year old arriving from countries with risk for YF virus transmission; this includes >12-hour airport transits or layovers in countries with risk for YF virus transmission. 1
Yellow Fever - CDC Yellow Book
Avoid contaminated water
Leptospirosis
How most people get sick (most common modes of transmission)
- Touching urine or other body fluids from an animal infected with leptospirosis
- Swimming or wading in urine-contaminated fresh water, or contact with urine-contaminated mud
- Drinking water or eating food contaminated with animal urine
- Avoid contaminated water and soil
- Avoid floodwater
Clinical Guidance
Avoid bug bites, chagas disease (american trypanosomiasis).
- Accidentally rub feces (poop) of the triatomine bug into the bug bite, other breaks in the skin, your eyes, or mouth
- From pregnant woman to her baby, contaminated blood products (transfusions), or contaminated food or drink.
- Avoid Bug Bites
Chagas disease
- Mosquito bite
Leishmaniasis
- Sand fly bite
- An infected pregnant woman can spread it to her unborn baby
Airborne & droplet
- Breathing in air or accidentally eating food contaminated with the urine, droppings, or saliva of infected rodents
- Bite from an infected rodent
- Less commonly, being around someone sick with hantavirus (only occurs with Andes virus)
- Avoid rodents and areas where they live
- Avoid sick people
Tuberculosis (TB)
- Breathe in TB bacteria that is in the air from an infected and contagious person coughing, speaking, or singing.
Learn actions you can take to stay healthy and safe on your trip. Vaccines cannot protect you from many diseases in Guatemala, so your behaviors are important.
Eat and drink safely
Food and water standards around the world vary based on the destination. Standards may also differ within a country and risk may change depending on activity type (e.g., hiking versus business trip). You can learn more about safe food and drink choices when traveling by accessing the resources below.
- Choose Safe Food and Drinks When Traveling
- Water Treatment Options When Hiking, Camping or Traveling
- Global Water, Sanitation and Hygiene (WASH)
- Avoid Contaminated Water During Travel
You can also visit the Department of State Country Information Pages for additional information about food and water safety.
Prevent bug bites
Bugs (like mosquitoes, ticks, and fleas) can spread a number of diseases in Guatemala. Many of these diseases cannot be prevented with a vaccine or medicine. You can reduce your risk by taking steps to prevent bug bites.
What can I do to prevent bug bites?
- Cover exposed skin by wearing long-sleeved shirts, long pants, and hats.
- Use an appropriate insect repellent (see below).
- Use permethrin-treated clothing and gear (such as boots, pants, socks, and tents). Do not use permethrin directly on skin.
- Stay and sleep in air-conditioned or screened rooms.
- Use a bed net if the area where you are sleeping is exposed to the outdoors.
What type of insect repellent should I use?
- FOR PROTECTION AGAINST TICKS AND MOSQUITOES: Use a repellent that contains 20% or more DEET for protection that lasts up to several hours.
- Picaridin (also known as KBR 3023, Bayrepel, and icaridin)
- Oil of lemon eucalyptus (OLE) or para-menthane-diol (PMD)
- 2-undecanone
- Always use insect repellent as directed.
What should I do if I am bitten by bugs?
- Avoid scratching bug bites, and apply hydrocortisone cream or calamine lotion to reduce the itching.
- Check your entire body for ticks after outdoor activity. Be sure to remove ticks properly.
What can I do to avoid bed bugs?
Although bed bugs do not carry disease, they are an annoyance. See our information page about avoiding bug bites for some easy tips to avoid them. For more information on bed bugs, see Bed Bugs .
For more detailed information on avoiding bug bites, see Avoid Bug Bites .
Stay safe outdoors
If your travel plans in Guatemala include outdoor activities, take these steps to stay safe and healthy during your trip.
- Stay alert to changing weather conditions and adjust your plans if conditions become unsafe.
- Prepare for activities by wearing the right clothes and packing protective items, such as bug spray, sunscreen, and a basic first aid kit.
- Consider learning basic first aid and CPR before travel. Bring a travel health kit with items appropriate for your activities.
- If you are outside for many hours in heat, eat salty snacks and drink water to stay hydrated and replace salt lost through sweating.
- Protect yourself from UV radiation : use sunscreen with an SPF of at least 15, wear protective clothing, and seek shade during the hottest time of day (10 a.m.–4 p.m.).
- Be especially careful during summer months and at high elevation. Because sunlight reflects off snow, sand, and water, sun exposure may be increased during activities like skiing, swimming, and sailing.
- Very cold temperatures can be dangerous. Dress in layers and cover heads, hands, and feet properly if you are visiting a cold location.
Stay safe around water
- Swim only in designated swimming areas. Obey lifeguards and warning flags on beaches.
- Practice safe boating—follow all boating safety laws, do not drink alcohol if driving a boat, and always wear a life jacket.
- Do not dive into shallow water.
- Do not swim in freshwater in developing areas or where sanitation is poor.
- Avoid swallowing water when swimming. Untreated water can carry germs that make you sick.
- To prevent infections, wear shoes on beaches where there may be animal waste.
Leptospirosis, a bacterial infection that can be spread in fresh water, is found in Guatemala. Avoid swimming in fresh, unchlorinated water, such as lakes, ponds, or rivers.
Keep away from animals
Most animals avoid people, but they may attack if they feel threatened, are protecting their young or territory, or if they are injured or ill. Animal bites and scratches can lead to serious diseases such as rabies.
Follow these tips to protect yourself:
- Do not touch or feed any animals you do not know.
- Do not allow animals to lick open wounds, and do not get animal saliva in your eyes or mouth.
- Avoid rodents and their urine and feces.
- Traveling pets should be supervised closely and not allowed to come in contact with local animals.
- If you wake in a room with a bat, seek medical care immediately. Bat bites may be hard to see.
All animals can pose a threat, but be extra careful around dogs, bats, monkeys, sea animals such as jellyfish, and snakes. If you are bitten or scratched by an animal, immediately:
- Wash the wound with soap and clean water.
- Go to a doctor right away.
- Tell your doctor about your injury when you get back to the United States.
Consider buying medical evacuation insurance. Rabies is a deadly disease that must be treated quickly, and treatment may not be available in some countries.
Reduce your exposure to germs
Follow these tips to avoid getting sick or spreading illness to others while traveling:
- Wash your hands often, especially before eating.
- If soap and water aren’t available, clean hands with hand sanitizer (containing at least 60% alcohol).
- Don’t touch your eyes, nose, or mouth. If you need to touch your face, make sure your hands are clean.
- Cover your mouth and nose with a tissue or your sleeve (not your hands) when coughing or sneezing.
- Try to avoid contact with people who are sick.
- If you are sick, stay home or in your hotel room, unless you need medical care.
Avoid sharing body fluids
Diseases can be spread through body fluids, such as saliva, blood, vomit, and semen.
Protect yourself:
- Use latex condoms correctly.
- Do not inject drugs.
- Limit alcohol consumption. People take more risks when intoxicated.
- Do not share needles or any devices that can break the skin. That includes needles for tattoos, piercings, and acupuncture.
- If you receive medical or dental care, make sure the equipment is disinfected or sanitized.
Know how to get medical care while traveling
Plan for how you will get health care during your trip, should the need arise:
- Carry a list of local doctors and hospitals at your destination.
- Review your health insurance plan to determine what medical services it would cover during your trip. Consider purchasing travel health and medical evacuation insurance.
- Carry a card that identifies, in the local language, your blood type, chronic conditions or serious allergies, and the generic names of any medications you take.
- Some prescription drugs may be illegal in other countries. Call Guatemala’s embassy to verify that all of your prescription(s) are legal to bring with you.
- Bring all the medicines (including over-the-counter medicines) you think you might need during your trip, including extra in case of travel delays. Ask your doctor to help you get prescriptions filled early if you need to.
Many foreign hospitals and clinics are accredited by the Joint Commission International. A list of accredited facilities is available at their website ( www.jointcommissioninternational.org ).
In some countries, medicine (prescription and over-the-counter) may be substandard or counterfeit. Bring the medicines you will need from the United States to avoid having to buy them at your destination.
Malaria is a risk in some parts of Guatemala. If you are going to a risk area, fill your malaria prescription before you leave, and take enough with you for the entire length of your trip. Follow your doctor’s instructions for taking the pills; some need to be started before you leave.
Select safe transportation
Motor vehicle crashes are the #1 killer of healthy US citizens in foreign countries.
In many places cars, buses, large trucks, rickshaws, bikes, people on foot, and even animals share the same lanes of traffic, increasing the risk for crashes.
Be smart when you are traveling on foot.
- Use sidewalks and marked crosswalks.
- Pay attention to the traffic around you, especially in crowded areas.
- Remember, people on foot do not always have the right of way in other countries.
Riding/Driving
Choose a safe vehicle.
- Choose official taxis or public transportation, such as trains and buses.
- Ride only in cars that have seatbelts.
- Avoid overcrowded, overloaded, top-heavy buses and minivans.
- Avoid riding on motorcycles or motorbikes, especially motorbike taxis. (Many crashes are caused by inexperienced motorbike drivers.)
- Choose newer vehicles—they may have more safety features, such as airbags, and be more reliable.
- Choose larger vehicles, which may provide more protection in crashes.
Think about the driver.
- Do not drive after drinking alcohol or ride with someone who has been drinking.
- Consider hiring a licensed, trained driver familiar with the area.
- Arrange payment before departing.
Follow basic safety tips.
- Wear a seatbelt at all times.
- Sit in the back seat of cars and taxis.
- When on motorbikes or bicycles, always wear a helmet. (Bring a helmet from home, if needed.)
- Avoid driving at night; street lighting in certain parts of Guatemala may be poor.
- Do not use a cell phone or text while driving (illegal in many countries).
- Travel during daylight hours only, especially in rural areas.
- If you choose to drive a vehicle in Guatemala, learn the local traffic laws and have the proper paperwork.
- Get any driving permits and insurance you may need. Get an International Driving Permit (IDP). Carry the IDP and a US-issued driver's license at all times.
- Check with your auto insurance policy's international coverage, and get more coverage if needed. Make sure you have liability insurance.
- Avoid using local, unscheduled aircraft.
- If possible, fly on larger planes (more than 30 seats); larger airplanes are more likely to have regular safety inspections.
- Try to schedule flights during daylight hours and in good weather.
Medical Evacuation Insurance
If you are seriously injured, emergency care may not be available or may not meet US standards. Trauma care centers are uncommon outside urban areas. Having medical evacuation insurance can be helpful for these reasons.
Helpful Resources
Road Safety Overseas (Information from the US Department of State): Includes tips on driving in other countries, International Driving Permits, auto insurance, and other resources.
The Association for International Road Travel has country-specific Road Travel Reports available for most countries for a minimal fee.
For information traffic safety and road conditions in Guatemala, see Travel and Transportation on US Department of State's country-specific information for Guatemala .
Maintain personal security
Use the same common sense traveling overseas that you would at home, and always stay alert and aware of your surroundings.
Before you leave
- Research your destination(s), including local laws, customs, and culture.
- Monitor travel advisories and alerts and read travel tips from the US Department of State.
- Enroll in the Smart Traveler Enrollment Program (STEP) .
- Leave a copy of your itinerary, contact information, credit cards, and passport with someone at home.
- Pack as light as possible, and leave at home any item you could not replace.
While at your destination(s)
- Carry contact information for the nearest US embassy or consulate .
- Carry a photocopy of your passport and entry stamp; leave the actual passport securely in your hotel.
- Follow all local laws and social customs.
- Do not wear expensive clothing or jewelry.
- Always keep hotel doors locked, and store valuables in secure areas.
- If possible, choose hotel rooms between the 2nd and 6th floors.
Healthy Travel Packing List
Use the Healthy Travel Packing List for Guatemala for a list of health-related items to consider packing for your trip. Talk to your doctor about which items are most important for you.
Why does CDC recommend packing these health-related items?
It’s best to be prepared to prevent and treat common illnesses and injuries. Some supplies and medicines may be difficult to find at your destination, may have different names, or may have different ingredients than what you normally use.
If you are not feeling well after your trip, you may need to see a doctor. If you need help finding a travel medicine specialist, see Find a Clinic . Be sure to tell your doctor about your travel, including where you went and what you did on your trip. Also tell your doctor if you were bitten or scratched by an animal while traveling.
If your doctor prescribed antimalarial medicine for your trip, keep taking the rest of your pills after you return home. If you stop taking your medicine too soon, you could still get sick.
Malaria is always a serious disease and may be a deadly illness. If you become ill with a fever either while traveling in a malaria-risk area or after you return home (for up to 1 year), you should seek immediate medical attention and should tell the doctor about your travel history.
For more information on what to do if you are sick after your trip, see Getting Sick after Travel .
Map Disclaimer - The boundaries and names shown and the designations used on maps do not imply the expression of any opinion whatsoever on the part of the Centers for Disease Control and Prevention concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Approximate border lines for which there may not yet be full agreement are generally marked.
Other Destinations
If you need help finding travel information:
Message & data rates may apply. CDC Privacy Policy
File Formats Help:
- Adobe PDF file
- Microsoft PowerPoint file
- Microsoft Word file
- Microsoft Excel file
- Audio/Video file
- Apple Quicktime file
- RealPlayer file
- Zip Archive file
How does mpox spread and what is the risk to the rest of the world?
Africa's top health body has declared a public health emergency after more than 15,600 cases and 537 deaths were reported on the continent this year.
Thursday 15 August 2024 11:06, UK
Please use Chrome browser for a more accessible video player

Mpox has been declared a global emergency by the World Health Organization (WHO), with a new strain spreading across Africa at an alarming rate.
Officials announced on Wednesday that an outbreak of the strain in the Democratic Republic of the Congo was now a "public health emergency of international concern".
It is the second time in three years that the WHO has designated an mpox epidemic as a global emergency.
It comes as the number of cases reported so far this year has already exceeded last year's total, with more than 15,600 cases and 537 deaths according to the WHO.

But what is mpox, what are the symptoms, how is it treated and what's being done about the outbreak?
The viral disease occurs mostly in central and western Africa. The recent outbreak has spread to 13 African countries, including some that have never reported mpox cases before.
It was first identified in laboratory monkeys, according to the US Centers for Disease Control and Prevention (CDC).
It used to be known as monkeypox, but was renamed in 2022 by the World Health Organisation (WHO) after receiving complaints that the original name was "racist and stigmatising".

Most cases are mild but it can be deadly.
The disease spreads through close contact with infected people, including via sex, with the latest outbreak in the continent beginning with the spread of an endemic strain known as Clade 1.
The new variant that has emerged, known as Clade 1b, appears to spread more easily through close contact, particularly among children.
Jean Claude Udahemuka, from the University of Rwanda, said last month that Clade 1b is "undoubtedly the most dangerous so far of all the known strains of mpox".

What are the symptoms?
Common symptoms of mpox are a skin rash or pus-filled lesions which can last two to four weeks.
The rashes can be located anywhere on the body and some people may only have one, while others can have hundreds or more.
These are other symptoms listed by the CDC:
- Swollen lymph nodes
- Muscle aches and backache
- Respiratory symptoms (e.g., sore throat, nasal congestion, or cough)
The WHO says people may start to feel unwell before they get a rash or skin lesions, while for others the skin symptoms can be the first or only sign.

The new strand has the same symptoms as others but they are more severe, according to Leandre Murhula Masirika, a research coordinator in South Kivu province.
An analysis of patients hospitalised from October to January in eastern Congo suggested the new form of mpox initially caused milder symptoms and lesions mostly on the genitals, making it harder to spot.
How is it treated?
Currently there is no treatment approved specifically for mpox infections, according to the CDC.
It says that for most patients with mpox who have intact immune systems and don't have a skin disease, supportive care and pain control will help them recover without medical treatment.
However, a two-dose vaccine has been developed to protect against the virus, which is widely available in Western countries but not in Africa.
Scientists from the Africa Centres for Disease Control and Prevention (Africa CDC) say they need more than 10 million vaccine doses but only 200,000 are available.
How did things get worse in Africa?
Mpox has been endemic in parts of Africa for decades after it was first detected in humans in DR Congo in 1970.
But the Clade 1b strain first emerged in September among sex workers in the DRC mining town of Kamituga, about 170 miles (273km) from the border with Rwanda .
Africa CDC has said 96% of all cases and deaths were in Democratic Republic of Congo (DRC), but it has also spread to neighbouring countries, with 18 nations reporting cases.
Keep up with all the latest news from the UK and around the world by following Sky News
Could this affect the UK and the rest of the world?
A milder version of the virus spread to more than 100 countries in 2022, largely through sexual contact, prompting the WHO to declare a public health emergency of international concern on 23 July 2022 - its highest level of alert.
A total of 2,137 cases had been confirmed in the UK at that stage, but by 31 December 2022 that number had soared to 3,732 cases - 3,553 were in England, 34 in Northern Ireland, 97 in Scotland and 48 in Wales.
The WHO ended the emergency 10 months later, saying the health crisis had come under control.
Infections have since slowed down significantly in the UK, with only 239 cases detected in 2023 and 2024 (up to 30 June) - 225 in England, nine in Scotland, four in Northern Ireland and one in Wales.
Be the first to get Breaking News
Install the Sky News app for free

There were no reported deaths in the UK during the entire outbreak.
Maria Van Kerkhove, who leads WHO's outbreak department, has called for an urgent response.
"We do not want the world to sit and watch and wait," she said. "The time [to act] is now."
Related Topics

IMAGES
COMMENTS
Fever in the returning traveler is an evolving clinical challenge, with respect to both the infections responsible for the fever and the sources and quality of information available to assist the ...
CDC Yellow Book 2024. Fever often accompanies serious illness in returned travelers. The most common life-threatening tropical disease associated with fever in returned travelers is malaria. Because an increased temperature can signal a rapidly progressive infection, initiate early evaluation, especially in people who have visited areas with ...
The evaluation of fever in returned travelers should focus on the possible infections given the patient's clinical findings, travel geography, administration (if any) of vaccinations and malaria chemoprophylaxis, the nature and timeframe of potential exposure (s), and the incubation period (s) of the relevant possible infections ( table 1) [ 1 ...
Salient points of the history of present illness and the travel and medical history, descriptions of common nonfebrile syndromes, and initial management steps are outlined below. The differential diagnosis and management for a traveler with fever (or febrile syndrome) is discussed in detail in Sec. 11, Ch. 4, Fever in the Returned Traveler.
Dengue virus: 6% of all fever and 18% of undifferentiated fever from endemic regions in returned travelers primarily from Southeast Asia and The Americas. Zika virus often co-circulates with Dengue and Chikungunya viruses but the proportion of febrile illnesses due to this virus is unknown.
Once routine infections have been considered, the differential diagnosis should be expanded to include travel-related infections. The most serious cause of fever in travelers returning from the ...
If the patient has fever without a focus and a tropical infection is suspected, malaria, dengue fever, and typhoid fever are common causes.
A lack of pre-travel counseling was associated with acquisition of a febrile illness abroad, as was visiting friends and/or relatives (VFR) travel. While there was no age bias in fever presentation, male travelers were more likely than female travelers to present with fever. In retrospective, questionnaire-based studies, the incidence of ...
For clinicians who are less familiar with travel-related pathology, however, it is important to highlight that approximately 90% of the febrile tropical illnesses are due to a rather limited set ...
There are different types of travel-associated infections that cause fever, along with other symptoms to include diarrhea, vomiting, rashes, and muscle aches. The most common of these include malaria, dengue, typhoid fever, and chikungunya.
Structured approach to fever after return from travel. Fever, defined as a body temperature ≥ 38.3 °C, is an important symptom in the returning traveler seeking medical attention, as it leads to hospitalization in about 30 % of cases ( 13) and may be indicative of severe disease ( 14 ).
A fever typically accompanies serious illnesses in returning travelers. Patients with a fever should be treated as moderately ill. One barrier to an accurate and early diagnosis of travel-related ...
This pathway should be used to guide the evaluation and treatment of patients with fever and recent travel. ≥ 38 °C in a traveler within 30 days of return to the U.S.
In this article, we discuss the epidemiology, evaluation, and management of specific travel related infections such as malaria, typhoid fever, dengue fever, viral hemorrhagic fever, rickettsiosis, leptospirosis, schistosomiasis, gastrointestinal, and respiratory infections.
Common tropical diseases found include malaria, dengue, enteric fever, rickettsial infections and respiratory infections. General practitioners should be alert to the public health implications of travel related diseases. Fever is an important and relatively common presentation of infections in returning travellers.
Overview Subjective illness is common after traveling and up to 10% of patients with recent international travel will seek healthcare. Remember, patients with recent travel are at risk for travel-related and non-travel related infections.
On This Page Infectious Agent Transmission Epidemiology Risk for Travelers Clinical Presentation Diagnosis Treatment Prevention International Certificate Of Vaccination Or Prophylaxis Vaccine Requirements Versus Recommendations INFECTIOUS AGENT: Yellow fever virus ENDEMICITY Sub-Saharan Africa Tropical South America TRAVELER CATEGORIES AT GREATEST RISK FOR EXPOSURE & INFECTION Unimmunized ...
Some of the most common travel-related infections include: Avian flu: Avian, or bird flu is currently rare among travelers, but the virus can cause eye infections, flu-like symptoms, pneumonia and death. Cholera: This bacteria spread by ingesting contaminated water or food. Cholera causes diarrhea, nausea and vomiting.
Recent findings: The destination of travel determines the relative likelihood of the different major causes of fever. Systemic febrile illness occurs disproportionately among ill travelers returning from sub-Saharan Africa. Malaria remains the most important overall cause of systemic febrile illness in travelers to tropical regions; dengue ...
The Centers for Disease Control and Prevention issued a health alert on Friday warning of the increased risk of the Oropouche virus to travelers in the Americas, particularly those visiting the ...
As many as 34% of patients with recent travel history are diagnosed with routine infections, but serious infections such as malaria, enteric fever, and dengue fever should be on the differential diagnosis due the high morbidity and mortality in children. Keywords: Fever, Child, International travel, Tropical infections, Returning traveler.
Dengue is a viral illness that's spread by mosquitoes. Within a week of being bitten, symptoms may include high fever, headache, body aches, nausea and rash.
The Hawai'i Department of Health has identified a new case of travel-related dengue virus case on Oʻahu, bringing the number of total cases to 11 identified in the state in 2024 (one on Kauaʻi ...
Viral hemorrhagic fever (VHF) diseases are caused by 3 families ( Arenaviridae, Filoviridae, Flaviviridae) and 1 order ( Bunyavirales) of enveloped RNA viruses. Arenaviridae (arenaviruses) include Chapare, Guanarito, Junin, Lassa, and Lujo viruses; lymphocytic choriomeningitis virus (LCMV); and Machupo and Sabia viruses.
A seasonal respiratory virus named parvovirus B19 - sometimes also called fifth disease - is increasing in activity, the US Centers for Disease Control and Prevention warned Tuesday.
weeks before you travel to review country-specific travel information for the most up- to-date guidance on dengue risk and prevention measures for that country. Travelers returning from an area with risk of dengue should take steps to prevent mosquito bites for three weeks, and if symptoms of dengue develop within two weeks upon return,
Enteric fever is a foodborne infectious disease caused by Salmonella enterica serotypes Typhi and Paratyphi A, B and C. The high incidence in low income countries can increase the risk of disease in travelers coming from high income countries. Pre-travel ...
Europe's holiday destinations where risk of hot weather related death is at its highest have been revealed, from Greece to Italy and Spain.
Healthy Travel Packing List Use the Healthy Travel Packing List for Guatemala for a list of health-related items to consider packing for your trip. Talk to your doctor about which items are most important for you.
Africa's top health body has declared a public health emergency after more than 15,600 cases and 537 deaths were reported on the continent this year.